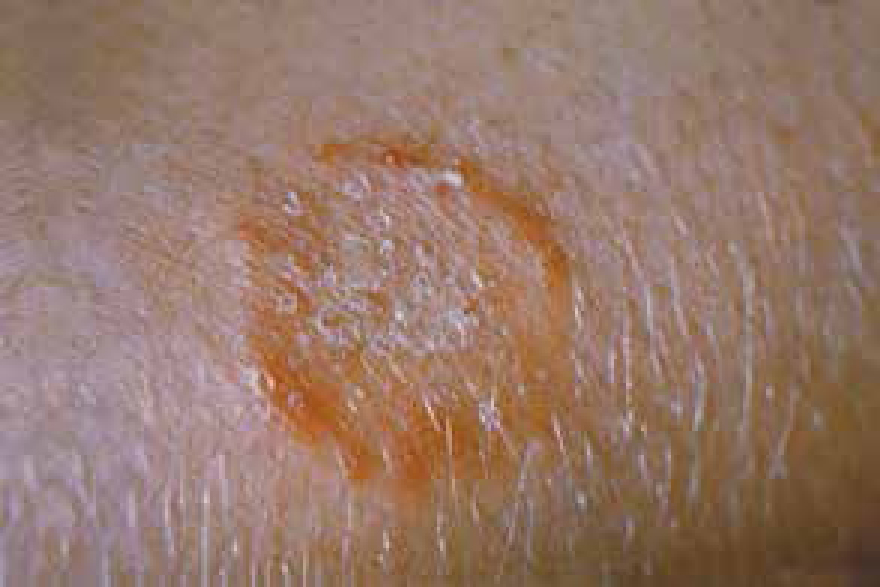
There are a number of skin conditions dermatologists see that many veterinary nurses (VNs) will not be aware of, however, dermatophytosis or ringworm is a skin disease commonly encountered in first opinion practice and often noted for its zoonotic nature (Duclos, 2012) (Figure 1).
VNs should consider how much they know about dermatophytosis and what they can do to assist the veterinary surgeon (VS) in the diagnosis and treatment of this relatively common condition. Despite being referred to as ringworm, this is not a parasite but a fungal infection caused typically by three species of fungus (dermatophytes) (Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes), which infect the hair, nail or skin (Gaudiano, 2005). This article aims to not only enlighten VNs on the condition, but to explore how this is an area in which the VN can play a pivotal role in patient outcome.
How VNs can help facilitate gathering a history
Gaudiano (2005) suggests a thorough history is essential for any good skin workup, and very often a 15 minute consult with a VS is not long enough for this to be achieved. Dermatology clinics with a dedicated VN are ideal for collecting a full clinical history with a dermatology questionnaire to go through with the client. A range of questions can be included on all aspects of skin health, lifestyle, breed, as well as a medical background which may pick up on immunosuppressive conditions, stressful home situations or drug therapy, which may predispose the animal to infection (Sierra et al, 2000; Duclos, 2012). Typical questions that can provide useful clues towards making a diagnosis of dermatophytosis might include levels of contact with other pets, asking if other pets in the household have been affected by skin conditions or if the owners have noticed any lesions on themselves. These are great markers for dermatophytosis, and can be obtained simply from a routine history (Gaudiano, 2005). A questionnaire is strongly recommended; whether the VN goes through it with the owner or gives it to them to complete before seeing the VS, it will aid the VS in achieving a diagnosis.
Physical and dermatological examination — and why the VN's pre-gathered history can help
It may seem like a full patient history is unnecessary when the VS can do a physical examination of lesions, but one of the challenges for VSs about dermatophytosis is its visual similarity to other conditions. Described as pleomorphic (meaning it can take different forms), the lesions can vary greatly, and animals may present with symptoms such as: scales; crusts; hair loss; erythema; papules; nodules; hyperpigmentation; with varying combinations (Duclos, 2012) (Figure 2). They can have the appearance of other conditions, and differential diagnoses include demodicosis, bacterial folliculitis, seborrheic complex, furuncolosis or an autoimmune disorder (Foster and Shaw, 2014; Paterson, 2017). Having a thorough history alongside their examination can help the VS eliminate other conditions.

Diagnostic tests; where a VNs time is invaluable
General workup
Tests are an area the VN can get involved with, starting with general dermatology workups. The full range of skin tests are always advised to ensure elimination of other conditions, even if they are secondary to an underlying problem (Gaudiano, 2005). Skin scrapes and/or hair plucks, for example, can rule out other suspected conditions such as demodicosis; while cytology, in the form of tape strips, impression smears and swabs of primary lesions, can indicate bacterial or yeast infections (Prelaud et al, 2011). This article alone is not sufficient to cover the method for these tests, but it is important to note that they are all tests a VN can perform, where often the VS does not have the time (Robinson et al, 2014).
Wood's lamp
It is common in practice for VSs to use the Wood's lamp technique to look for ringworm — again this is something a VN can do. There are a few key points to note: make sure the room is dark and that the lamp is given at least 5 minutes to warm up; in addition using a lamp with a magnifier is helpful in finding any dermatophytes that are present (Gaudiano 2005). Also note: only M. canis will fluoresce; this makes up about 50–70% of dermatophytosis cases in dogs and cats, however it is not a certainty that all of these will fluoresce, and in addition, identifying dermatophytosis using this technique requires practice (Duclos, 2012). So, the Wood's lamp is far from an elimination test. A positive test is indicated by an apple green fluorescence of the hair shaft; but care is required as creams and scale can fluoresce as well — amongst other things — leading to false positives (Caplan, 1967). It is still accepted as a good screening tool in combination with further testing (Bernard, 2013).
Microscopic examination
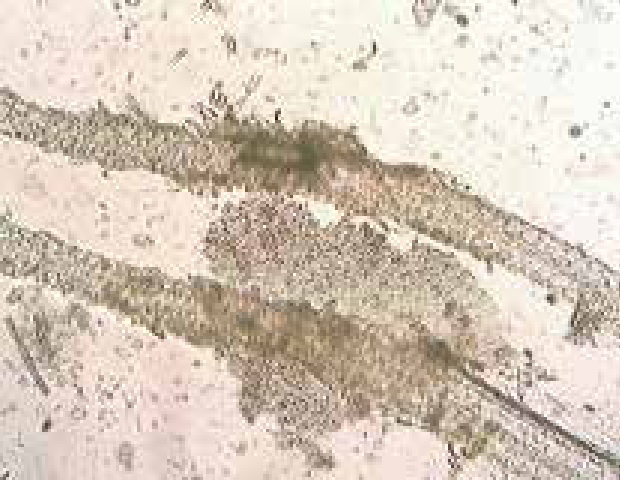
Microscopic examination of hair plucks or skin scrapes from the edge of lesions can certainly be done by a VN, looking for fungal hyphae or spores, especially after a suspected positive result with a Wood's lamp. This can be performed by plucking fluorescing hairs and mounting them onto a slide with liquid paraffin for examination; it should be noted this requires practice (Figure 3a) (Paterson, 2017).
Fungal culture
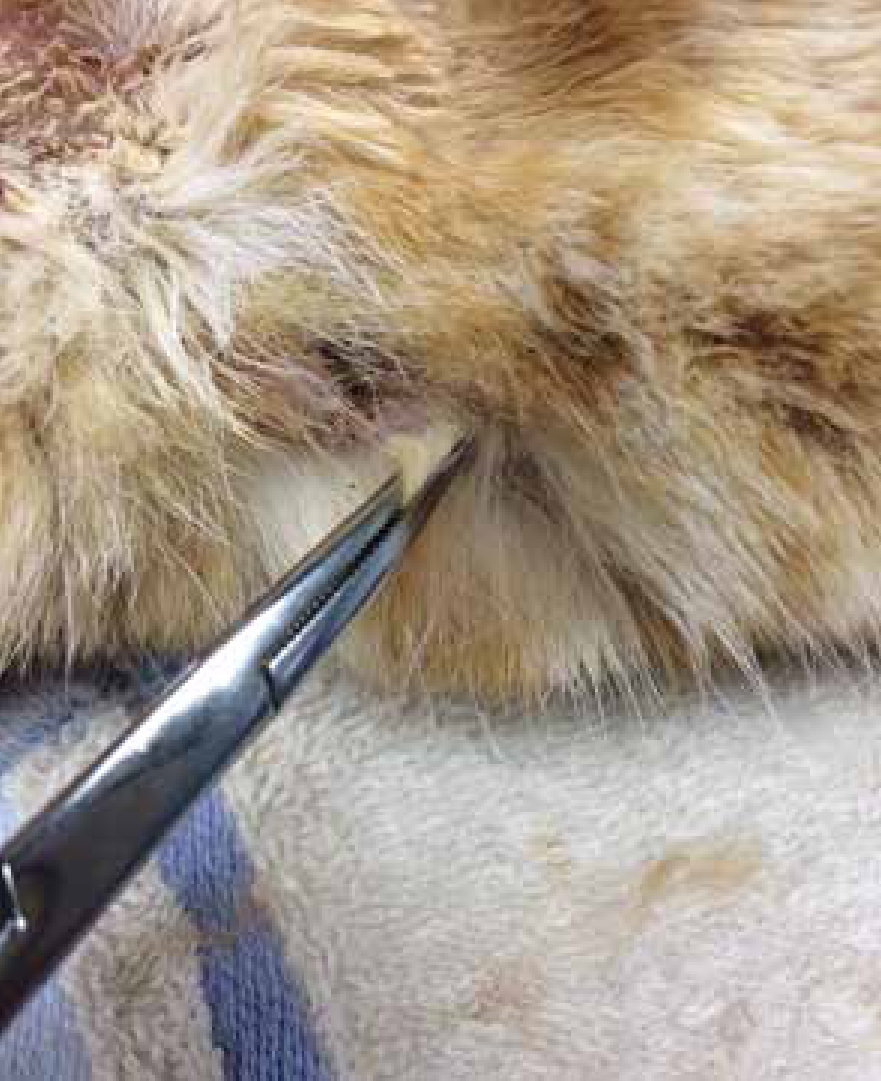
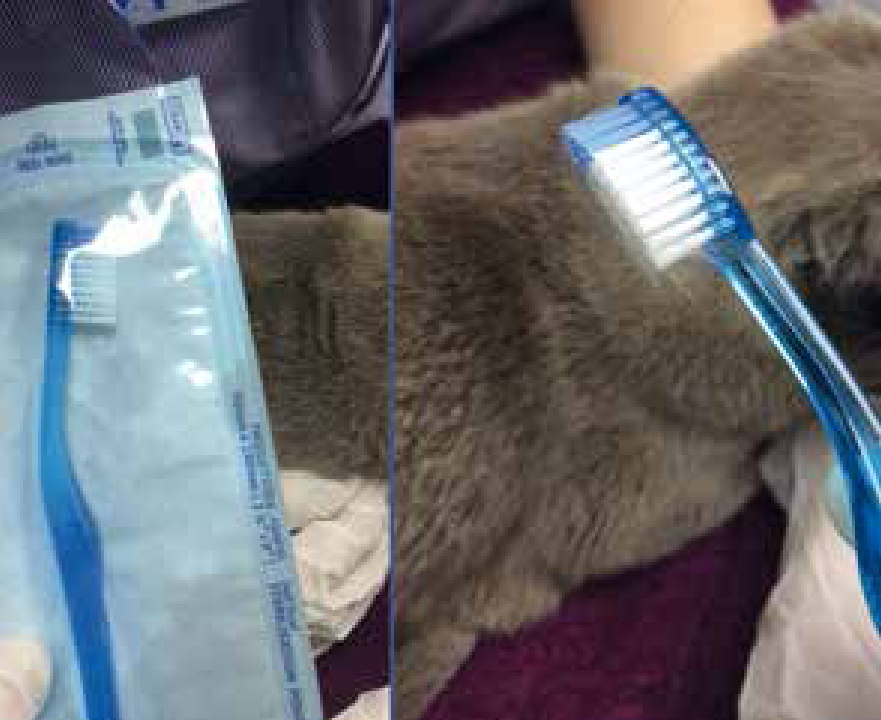
The best way to test for dermatophytosis is with culture (Duclos, 2012) and dermatophyte test medium (DTM) can be purchased to perform in-house dermatophyte cultures (Figure 3b); external labs also offer this service. It is important that samples are gathered correctly and with care (Sparkes et al, 1993), and as such this is another area VNs can be involved with. Hair can be plucked, preferably by rolling them out with a pair of forceps in order to retain the debris needed (Figure 4), from the edge of active lesions; dermatophyte spores if present will be found in this debris. Crusts can also be taken by scalpel scrapes from the same area of lesions, with several, if possible, being sampled (Gaudiano, 2005). If sampling companion animals that are not symptomatic, or a generalised infection is suspected, a Denman brush or toothbrush (Figure 5) can be used, combed through the hair for at least 2 minutes to take the sample. Brush non affected areas first and then lesions if present (Duclos, 2012).
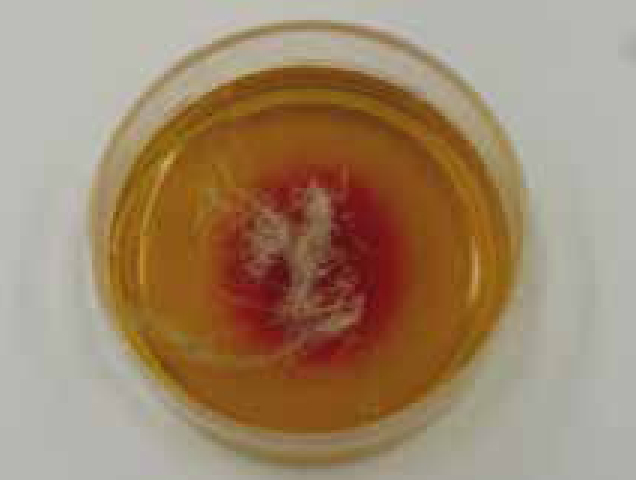
These DTM kits work by growing the culture in medium. The samples should be pressed but not imbedded into the medium (brushes pressed into firm contact), and then incubated with the lid loose at 26°C (Gaudiano, 2005). The dermatophyte grows and as it metabolises proteins, released alkaline metabolites which change the pH of the medium; pH indicators then change colour. For example DTM which is commonly used, changes to red (Figure 3b). This normally happens within 10 days, which should be noted as non pathogenic fungi (not ringworm) which prefer a carbohydrate substrate will start consuming proteins if these run out of carbohydrates, causing alkalisation and a late colour change; a late colour change may, therefore, be a false positive (Salkin, 1973).
Other things to be aware of with in-house culture include difficulty with sampling, incubation, and interpretation; these mean that false negatives and positives are common (Sparkes et al, 1993). In addition, the kits do not detect all species. If dermatophytes do grow, it is best to examine colonies at the end of culture to identify the exact type present. This is done by sampling the colony from the culture medium with clear tape, then sticking it down onto a slide with a drop of lactophenol cotton blue stain, then another drop of this on top of the tape followed by a coverslip; the colonies are then examined for shape and colour. The colour is affected by DTM agar however, as is the colony shape — so this is another limiting factor in-house, unless using a split plate with Sabaruraud's medium. In-house can, therefore, be challenging and best practice is to send the samples to an external laboratory for interpretation (Duclos, 2012). If speed is of concern laboratories often now report the positive (or negative) first, when the colour change occurs, then the type later once the colony has grown; the initial report is usually back within 3–4 weeks.
Some animals may test positive for culture, but not actually be infected, instead acting as a transient mechanical carrier (Moriello et al, 2017). This culture result, along with the patient history, will enable the VS to determine the overall infection status of each pet. This is particularly important in multi-pet households, where all in-contact pets should be cultured and then treated where positive (Duclos, 2012). An informed VN may be able to offer guidance and information on testing methods to the pet owner so they understand how a diagnosis is being achieved, and why tests may need to be performed on other pets.
Treatment, and how a VN can help owners
In dermatophytosis the VN plays a vital role in educating and advising clients on treatment regimens to ensure a high level of owner compliance. In addition, as a zoonotic disease it is always essential that owners are made aware of the potential for contagion (Paterson, 2017).
Dermatophytosis is a self-limiting disease and will resolve within a few months in a patient with a healthy immune system without treatment (Paterson, 2017). The main problem with dermatophytosis is that it is contagious and will spread to other areas of the patient, to other patients and to humans (Moriello et al, 2017). Therefore, treatment is used to speed up the resolution of the disease, preventing it spreading across the patient and to other people and animals (Duclos, 2012).
Systemic treatment
The VS will always decide on the treatment options, but it is vital the VN has an understanding of them. They will generally choose one of three oral treatments to target the active site of the fungal infection, helping to prevent the spread of further lesions (Paterson, 2017). Itraconazole is the most popular and should be given with a meal; it can be hepatotoxic so bloods may be done at the start and possibly during therapy, something a VN can do while also explaining the reasons for this to the client. Recommended protocols vary, and those provided by Carlotti (2010) and Newbury et al (2011) are often used. Carlotti suggests 5 mg/kg orally for a week on and a week off until mycological cure is obtained; in his study this was at 56 days (Carlotti et al, 2010). Newbury et al (2011) used itraconazole at a dose of 10 mg/kg daily for 21 days with either lime sulphur or a chlorhexidine/miconazole product topically (see below) twice weekly. Mycological cure was achieved in 36 days (Newbury et al, 2011). In both cases the treatment took some time to achieve cure, and it is worth informing owners that this may be the case, but that treatment regimens must be adhered to.
Another common choice is terbinafine, this is is not licensed for the treatment of dermatophytosis in dogs and cats; again pre bloods are advised. It can cause vomiting and carries a risk of teratogenicity (Carlotti, 2010; Newbury et al, 2011).
Topical therapy: general considerations
When it comes to treatment in dermatology, the area a VN can really contribute to is topical therapy, both in aiding compliance by discussing it with the owner and also on occasion in application of the product. With dermatophytosis start by discussing the principle of topical therapy with the owner, so they are aware that topical treatment is critical to reduce the risk of spread, by removing and neutralising spores on the coat (Duclos, 2012). Transmission of dermatophytosis occurs via direct contact with infective material; topical therapy acts to decrease the infectious, contagious, and zoonotic risks associated with this disease by disinfecting the hair coat and minimising contamination of the environment (Paterson, 2017).
Clipping the coat prior to application of treatment is controversial (Bernard, 2013), although clipping 5 cm around lesions seems to be generally suggested to remove contaminant and help the topical treatment penetrate (Moriello et al, 2017). Whole body clips in a matted cat may be needed, but otherwise these are not as suggested as they once were (Paterson, 2017). However, if the VS does suggest clipping, the VN will need to perform this and it is essential that personal protective clothing is worn, followed by thorough disinfection of the environment (see below), and disinfection of the clippers. If thorough disinfection is not achieved, other patients and staff are at risk of catching dermatophytosis due to its contagious nature (Gaudiano, 2005).
Topical therapy: treatments
There are three main types of topical therapy currently recommended: enilconazole (Imaverol, Elanco), which is a dip that can leave the coat quite greasy and cause a reaction in cats; lime sulphur (Lime Sulfur Dip, Vetoquinol, USA) which smells like rotten egg and stains many things yellow and is currently not available in the UK; and a miconazole/chlorhexidine shampoo (Malaseb, Dechra) (Perrins and Bond, 2003). The latter is most popular in general practice, however lime sulphur or enilconazole have been shown to be most effective in recent studies (Moriello et al, 2017). All of these should be used twice weekly and have their frustrations, so the VN spending time with the owner talking through application is essential to aid compliance. There are also creams and ointments (clotrimazole, miconazole and enilconazole); these are not reccomended for sole therapy, but as concurrent treatments to systemic therapy (Moriello et al, 2017).
Preventing spread of disease
Other pets
Another serious consideration with these cases is the need to screen all in-contact pets — these should be isolated if they test negative, and treated if they test positive (Duclos, 2012). This can be extremely difficult for clients due to the difficult nature of treatment as described previously, and the difficulty in isolating those not infected, especially where cats are involved, so talking through the contagious nature of the disease, the existence of latent carriers and risk of reinfection is critical.
The environment
Owners should be talked to at length about the need and method for cleaning the environment. This is primarily to prevent spores from the environment getting on the coat and causing false positives, as infection from the environment alone is rare. False positive fungal culture results lead to prolonged systemic and/or topical therapy and excessive confinement of pets (Paterson, 2017). That said decontamination is always advised by dermatologists (Moriello et al, 2017) and effectively consist of cleaning thoroughly with an appropriate disinfectant.
When cleaning the practice environment, thoroughly vacuum any hair clippings and dispose of the vacuum bag, clean and disinfect clipper blades (if you have been clipping) then scrub the area thoroughly, preferably with a detergent to begin with before disinfecting using bleach at concentration of at least 1:10 (Moriello, 2014). Then educate owners on following the same procedure at home. Talk to them about what things could act as fomites and on ensuring these are cleaned regularly. They must launder bedding at at least 60°C, along with thoroughly vacuuming furnishings and carpets (Gaudiano, 2005).
Follow up
The contagious nature of the disease means treatment can be both long term and very challenging, so support for the owners from the VN with repeat consultations and courtesy phone calls can help ensure the best chance of a resolution. Educate the client on the notion of confining infected pets, making it more likely for them to be able to decontaminate the environment, as this can be extremely challenging (special consideration should be given to infected kittens from a socialisation point of view) (Paterson, 2017). Duclos (2012) states spores can live in the environment for up to 2 years, so it is important to ensure the client is aware of this to help them understand the importance of decontamination, before asking again in the future how this is going, to help ensure it continues.
Clinical cure is defined as two negative cultures, 7 days apart and treatment should continue until this is achieved. If this is being adhered to the VN is essential in helping ensure compliance, as if symptoms stop prior to this, as with many conditions, this is often when the owner perceives the patient cured. Due process should be followed to ensure the patient is free of infection (Bernard, 2013).
Conclusion
Dermatophytosis is a challenging condition to treat and requires a thorough and methodical approach from the veterinary team as far as diagnosis and management is concerned to ensure the disease resolves. VNs play a vital role in helping the VS, the owner and the patient achieve a resolution and can be involved from the moment a case is suspected. An owner needs to follow the diagnostic and treatment plans directed by the VS, and are, in the author's experience, more likely to do so if they have had time and follow-up support from a VN who knows about the challenges of the condition; and a patient is far more likely to reach a resolution to their condition, without infecting everyone in their path, if the VN has facilitated the fundamentally simple concepts outlined in this article.

