A 1-year-old English Springer Spaniel was referred to the hospital, from the referring veterinary practice, with a history of sudden collapse and diarrhoea after a walk. On presentation, the patient was collapsed and poorly responsive. A brief history was obtained which showed no previous relevant medical history and the patient was up to date with vaccinations.
Patient signalment
- Species: Canine
- Breed: English Springer Spaniel
- Age: 1 year
- Sex: Male neutered
Major body systems assessment
On presentation, the patient was triaged to gauge his respiratory, cardiovascular and neurological status. The vital parameters were as follows:
- Cardiovascular system — sinus tachycardia (heart rate (HR) 160 beats per minute (bpm): normal range 60–120 bpm), very weak peripheral pulses, pale mucous membranes with a delayed capillary refill time (CRT) of 3–4 seconds (normal range 1–2 seconds)
- Respiratory system — respiratory rate (RR) was 36 breaths per minute (normal range 10–30 breaths per minutes), with minimal effort and thoracic auscultation showed normal bronchovesicular sounds
- Neurological system — collapsed with severely obtunded mentation.
Initial veterinary investigations
The patient was supplemented with oxygen therapy via a face mask while a 20-gauge intravenous catheter was placed in the right cephalic vein. Bolus intravenous fluid therapy (IVFT) was initiated, using Hartmanns, an isotonic crystalloid. To further evaluate the patient's clinical status, a blood sample was obtained from the jugular vein for an emergency database. A comprehensive blood profile was obtained including electrolytes, haematology, biochemistry and venous blood gas, results are shown in Table 1.
Table 1. Emergency database
| Profile | Value | Comparison to reference range |
|---|---|---|
| Lactate | 5.7 mmol/litre | ↑ |
| pH | 7.2 | ↓ |
| Base excess | -8.4 | ↓ |
| Total solids (TS) | 42.6 g/litre | ↓ |
| Packed cell volume (PCV) | 62% | ↑ |
| Glucose | 1.1 mmol | ↓ |
| Creatinine | 171 umol/litre | ↑ |
| Alanine transaminase (ALT) | 1127 iu/litre | ↑ |
| Alkaline phosphatase (ALP) | 226 iu/litre | ↑ |
| Total bilirubin | 62.6 | ↑ |
| Platelets | 35 x109/litre | ↓ |
| Blood type | DEA1 positive |
Further investigations of abdominal imaging and gastroscopy found evidence of portal hypertension and severe gastritis. Radiographs of the thorax showed no evidence of pleural disease or effusion, but the pulmonary vessels were narrowed, consistent with hypovolaemia. With respect to the patient's presenting clinical signs and investigations, the veterinary surgeon (VS) suspected that the patient was suffering from multiple organ dysfunction syndrome (MODS). This syndrome is characterised by ‘the presence of altered organ function in an acutely ill patient such that homeostasis cannot be maintained without medical intervention’ (Bone et al, 1992: 1646). This patient received intensive medical treatment, and the role of the registered veterinary nurse (RVN) remains at the forefront when caring for patients with challenging conditions such as this. This patient care report focuses on the key nursing interventions, under supervision of the VS, with a particular focus on fluid resuscitation, and the basic and advanced monitoring of vital signs. In order to cover all the aspects of nursing care that were implemented, a nursing care plan was used.
Discussion
MODS is a complex disease process that varies in its clinical course, but which results in derangements in cardiovascular, hepatic, neurological, haematological, pulmonary and renal function (Hackett, 2011). MODS is a complication that arises as a result of an overwhelming inflammatory response known as the systemic inflammatory response syndrome (SIRS) (Osterbur et al, 2014). Numerous inciting causes can lead to systemic inflammation and can include infectious and non-infectious causes (Moore, 2016). If inflammation becomes uncontrolled or dysregulated by ongoing injury or from additional physiological insults, progression to severe SIRS can occur, and if left untreated can progress to multiple organ dysfunction and death (Hackett, 2011). SIRS with signs of infection indicates sepsis. The hospitalisation form for this patient included a problem list based on this complex syndrome and included: disseminated intravascular coagulation (DIC); sepsis; acute respiratory distress syndrome (ARDS); acute kidney injury (AKI); cardiomyopathy; encephalopathy; acute neurological dysfunction; and gastrointestinal and hepatic dysfunction.
Specific nursing care
Haemodynamic resuscitation
Treating patients with MODS remains largely supportive with prioritisation of the most life-threatening aspects first. There were no signs of airway or breathing difficulties in this patient, therefore treatment was aimed at fluid resuscitation for the treatment of shock (Aldrich, 2009). Shock is a state of organ hypoperfusion which, if left untreated, leads to cellular hypoxia and organ failure (Wemple, 2010). The VS used Hartmann's, an isotonic crystalloid, as it was most readily available, is cheap, and immediately increases intravascular volume (Hardy, 2012). An initial bolus was given at a rate of 10 ml/kg over 15 minutes. As discussed by Boag and Hughes (2005) there is no standard formula for volume resuscitation, therefore, each patient should have an individualised plan, which aims to meet end points of resuscitation. Although the fluid choice and rate are the decision of the VS, monitoring and evaluating the patient's response to therapy is commonly the responsibility of the RVN. An initial assessment was made of the patient's heart rate, respiration rate, pulse quality, mucous membrane colour, CRT, and mentation, and was reported back to the VS. These are vital parameters which become compromised during hypovolaemic shock and are indicators of how well the body is responding to fluid bolus therapy. The patient's vital signs were monitored every 15 to 30 minutes and recorded on the hospitalisation form. Blood pressure is another crucial parameter to monitor in these patients, which will be discussed later. After the second bolus of fluid the patient's heart rate decreased to 120 bpm, CRT improved to 2 seconds, pulse quality became palpable and mucous membrane colour was pale pink. This indicated that the patient was responding to therapy; however, it is important for RVNs to stay vigilant and timely with their checks as over-perfusion is a concern in patients receiving high volumes of fluid over a short period (Aldrich, 2009). Signs of over-perfusion may be seen as limb oedema, fluid coming from the nose or heard on chest auscultation as chest crackles, which is associated with pulmonary oedema (Humm and Kellett-Gregory, 2016). Lactate was measured initially every 2 hours to help guide fluid therapy as hyperlactaemia is most commonly caused by tissue hypoxia as a result of anaerobic metabolism within tissues (Karagiannas et al, 2006).
Patients with decreased perfusion and shock are at risk of becoming hypothermic. The body responds to shock by causing peripheral vasoconstriction to protect vital organs which can cause hypotension, reduced cardiac output and subsequent organ damage if not rectified (Lock, 2015). A rectal temperature was first taken during fluid resuscitation and showed that the patient was hypothermic (36.6°C: normal range for dogs 37.9–39.9oC). On reflection, the author should have checked the temperature as soon as the animal arrived onto the ward, as it would have highlighted that preventative measures were required sooner. A multimodal approach, as advised by Archer (2007), was implemented by placing heat pads under the patient's blanket and using a warm air blanket (bair hugger™, 3M).
Basic monitoring
Patients with MODS require intensive monitoring involving one to one nursing care. Basic monitoring of the patient's cardiovascular, respiratory and neurological systems was performed hourly and included measuring heart rate, respiration rate and effort, pulse quality, mucous membrane colour, CRT and temperature (Breton, 2012). Because patients with MODS are at risk of developing sepsis, it is imperative that trends in these parameters are monitored closely and recorded appropriately. Signs of sepsis can present as tachycardia, tachypnoea, hyperthermia or hypothermia, decreased CRT and brick red mucous membrane colour (Breton, 2012). However, these signs can vary between canine and feline patients, with felines commonly becoming bradycardic in sepsis (Boller and Otto, 2009). The following day after being admitted, the patient's heart rate increased from 72 bpm to 118 bpm with bounding pulses and brick red mucous membranes. These signs are indicative of sepsis, which prompted diagnostic imaging to identify an underlying cause.
Patients with MODS are at risk of developing disseminated intravascular coagulation (DIC), which is a result of global widespread inflammation and activation of the coagulation cascade (Breton, 2012). The patient was monitored for early signs of DIC which included bleeding or bruising of injection sites, venepuncture and catheter sites, and petechiae on the gums, pinna or abdomen (Hackner, 2014). During hospitalisation, it was noted that the patient had developed petechiae on the gums and abdomen (Figure 1). The VS was alerted and a blood sample to check coagulation function was requested. The results showed off-scale high prothrombin time (PT) and activated partial thromboplastin time (aPTT), which are coagulation assays of secondary haemostasis. Primary haemostasis refers to the initial formation of a platelet clot and is dependent on normal platelet number and function (Herring and McMichael, 2012). Whereas, secondary haemostasis is the activation of the clotting cascade that results in the formation of thrombin, which converts fibrinogen to fibrin, and ultimately reinforcement of the platelet plug (Herring and McMichael, 2012). Secondary haemostatic disorders can be caused by inherited coagulation deficiencies, or from acquired defects such as liver failure, disseminated intravascular coagulopathy (DIC) or vitamin K deficiency (Herring and McMichael, 2012).
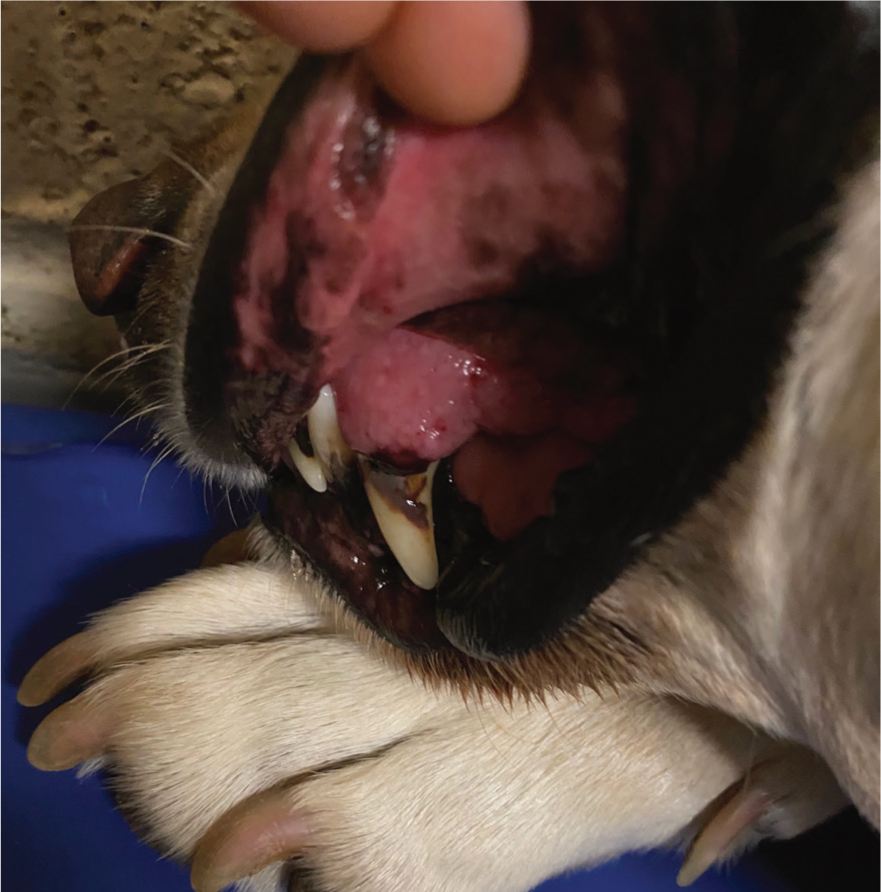
The suspected coagulopathy meant that the author was mindful not to take blood samples from the jugular vein as haemorrhage from the needle site is a concern in these patients and therefore, a pressure bandage needs to be applied, which is not possible with a jugular vein. As advised by Holt and Riley (2019), the cephalic vein was used instead, and a pressure bandage was applied directly after. Pictures were taken of the petechiae and recorded onto the patient's online flow chart so that they could be referred back to and used for comparison. The ‘problem list’ on the patient's hospitalisation chart was updated to include coagulopathy and ‘no further jugular samples’ was recorded under ‘critical’ so that other members of staff were aware. This patient received a fresh frozen plasma (FFP) transfusion for coagulation support. A reaction to FFP is unlikely to occur with this type of transfusion, however, the patient was monitored for signs of urticaria, vomiting and fever every 15 minutes for the first hour (Davidow, 2013). No adverse reactions were observed.
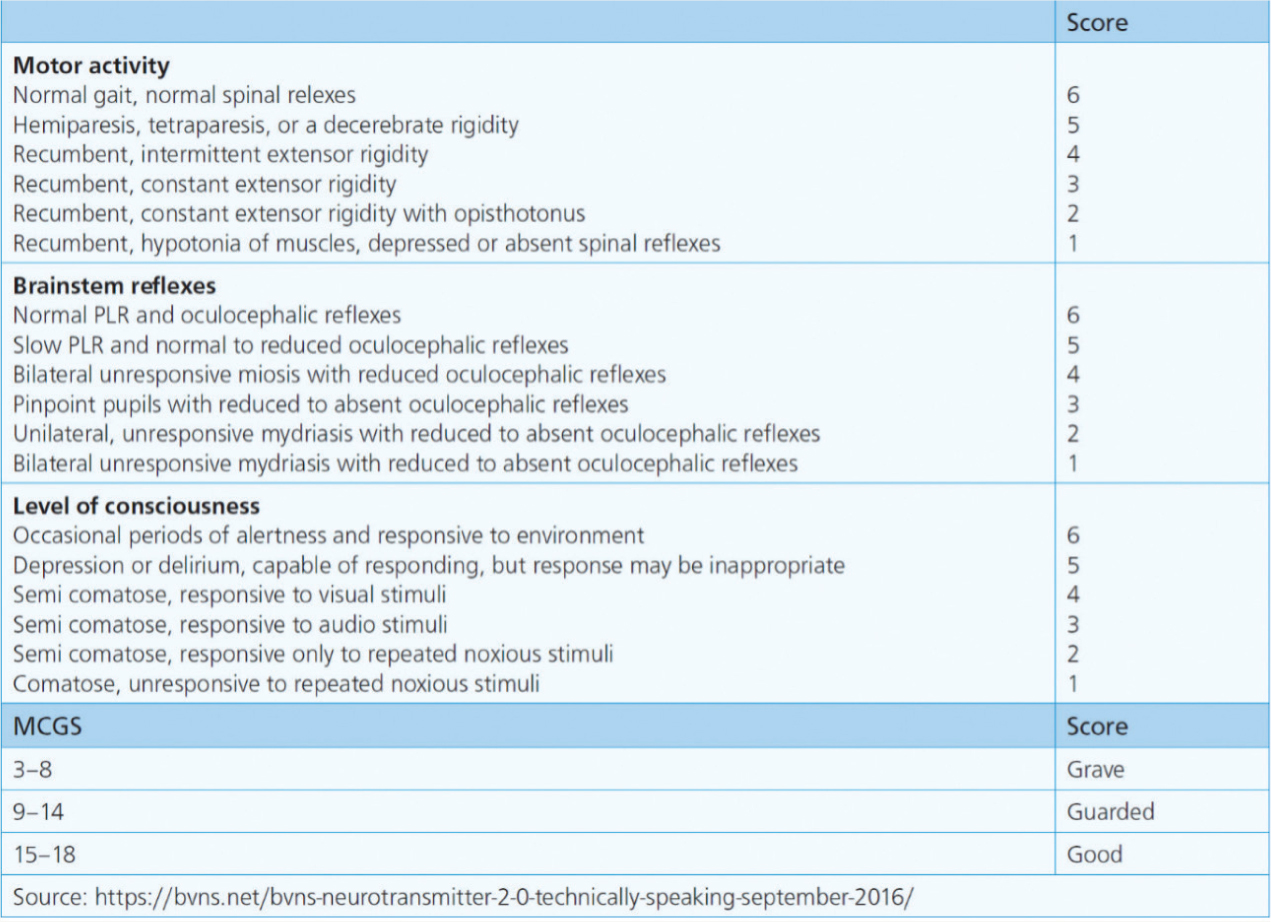
Mentation is an important component within the basic patient assessment. This patient required regular checks of responsiveness, pupil size and cranial nerve reflexes as these can indicate changes to the central nervous system (CNS), which can quickly advance to coma and death if left unnoticed (Humm and Kellett-Gregory, 2016). Alongside serial measurements of vital signs, the modified Glasgow coma scale (mGCS, Figure 2) would have been a useful tool for monitoring the severity and progression of the patient's neurological status. The mGCS comprises three categories: motor activity; brainstem reflexes; and level of consciousness. Each category is scored 1–6 with a total score of 3–18 (Elias et al, 2019). A lower score would indicate more severe neurological dysfunction.
Advanced monitoring
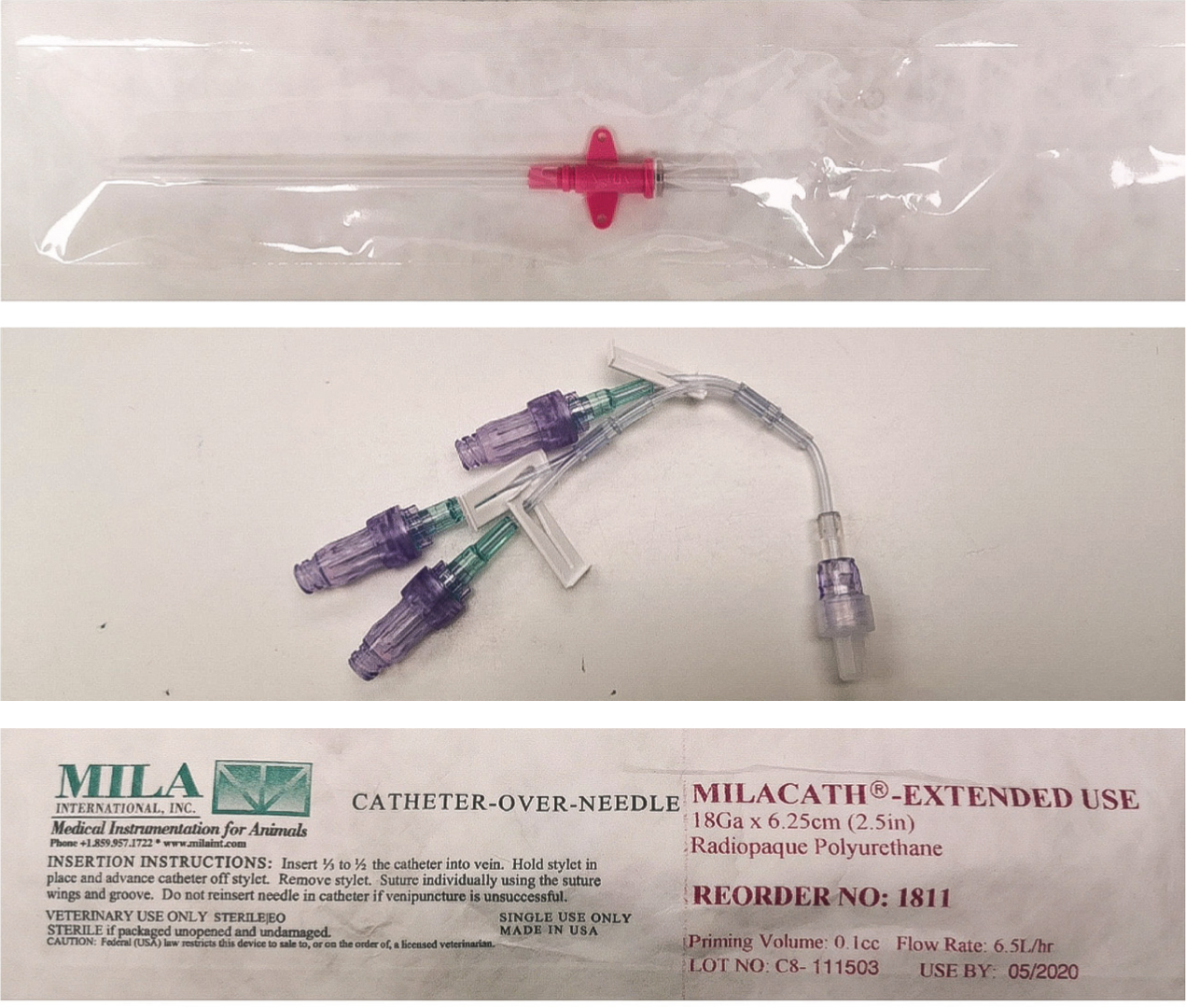
Numerous complications can arise from MODS, therefore, advanced monitoring equipment was implemented for verification of clinical findings. This included indirect blood pressure monitoring, blood glucose measurements, and blood gases measurements. It was predicted that multiple blood sampling would be required, therefore, a central venous line would have been desirable (Breton, 2012). Additionally, a central catheter would have guided fluid therapy as central venous pressure gives accurate measurements of vascular filling (Boag and Hughes, 2007). Prolonged coagulation times meant that a central line was contraindicated in this patient as bleeding is a complication during the initial placement (Kornbau et al, 2015). A long-term, large bore intravenous catheter with wings was inserted into the medial saphenous vein and sutured in place to prevent dislodgement (Figure 3). A multi-lumen extension tube was attached to the end of the catheter, which allowed for a mixture of fluid therapy, drug administration and blood sampling for 2 hourly lactate, electrolyte and blood gas analysis (Adamantos, 2018). The catheter was inserted while wearing sterile gloves as strict asepsis is crucial for the prevention of nosocomial infection in these patients (Mann, 2018). Prior to insertion, the area was clipped and a full surgical scrub using chlorhexidine gluconate in sterile water (1:1 dilution) was performed (Reynolds, 2019). The catheter was labelled with a ‘venous’ sticker so that other members of staff were cautious when administering medication or taking blood samples. This is crucial when arterial lines and multiple catheters are in situ to prevent accidental intra-arterial injection of drugs (Sen et al, 2005). The catheter was flushed every 6 hours to ensure patency and was inspected for signs of swelling, redness or dislodgement. The catheter was re-dressed every 12 hours and this was recorded on the patient's hospitalisation form (Ashby, 2017).
It is vital that patients with MODS have their blood pressure measured frequently as it can be used to recognise hypotension and hypoperfusion (Osterbur et al, 2014). Blood pressure can be measured directly via an arterial catheter or indirectly using non-invasive devices such as a Doppler machine or oscillometric device (Love and Harvey, 2006). As discussed by Pachtinger (2013), direct arterial measurement is considered gold standard as it allows for real time changes to be monitored, nevertheless, the disadvantages of arterial catheters include invasiveness, the need for technical skill with placement and familiarity with a transducer (Thomas and Boller, 2018). Additionally, it is deemed unsafe in patients with coagulopathy because of the associated complications, such as haemorrhage and haematoma formation (Bosiack et al, 2010). An oscillometric device was used as it provided non-invasive, automated blood pressure which yielded estimates of systolic arterial pressure (SAP), diastolic arterial pressure (DAP) and mean arterial pressure (MAP) readings (Acierno et al, 2013). Oscillometry was chosen over a Doppler device as a Doppler will give readings of SAP only. Despite fluid therapy, the patient's blood pressure was persistently low, requiring vasopressor support. The VS requested norepinephrine to be administered at a rate of 0.3 μg/kg/minute. ‘Notify’ levels were provided by the clinician so that they were alerted if SAP dropped below 100 mmHg or rose over 150 mmHg, DAP was <70 mmHg or >100 mmHg, and MAP <80 mmHg or >110 mmHg. This proved important as the SAP regularly dropped below 100 mmHg, requiring adjustment to the dose of norepinephrine from 0.3 to 0.6 μg/kg/minute, as instructed by the VS. As evidenced by Bosiack et al (2010), indirect blood pressure methods are considered less accurate than direct methods because of inconsistencies such as incorrect cuff selection, as falsely low readings can occur if the cuff is too big, or falsely high readings if too small (Sierra and Savino, 2015). The cuff chosen for the patient was in line with the accepted standards of cuff width, which should be approximately 40% of the circumference site (Skelding and Valverde, 2020).
Blood glucose was monitored in this patient as hypoglycaemia can be the result of impaired liver function and sepsis (Idowu and Heading, 2018). The patient was found to be severely hypoglycaemic (1.1 mmol/litre: normal range 3.3 mmol/litre to 6.2 mmol/litre), requiring emergency intervention to eliminate neuroglycopenic signs. The VS requested a glucose bolus at a dose rate of 0.5 ml/kg with 50% glucose, diluted with saline in a 1:3 ratio. Blood glucose measurements, using a glucometer, were initially monitored every 15 minutes and within 30 minutes, the patient's blood glucose had returned to slightly above the normal range (6.6 mmol/litre). Euglycaemia was achieved via a constant rate infusion (CRI) of 5% dextrose diluted in 0.9% saline. Hourly glucose measurements were recorded, and the VS was alerted if levels dropped below 4 mmol/litre or above 8 mmol/litre.
Recommendations for future practice
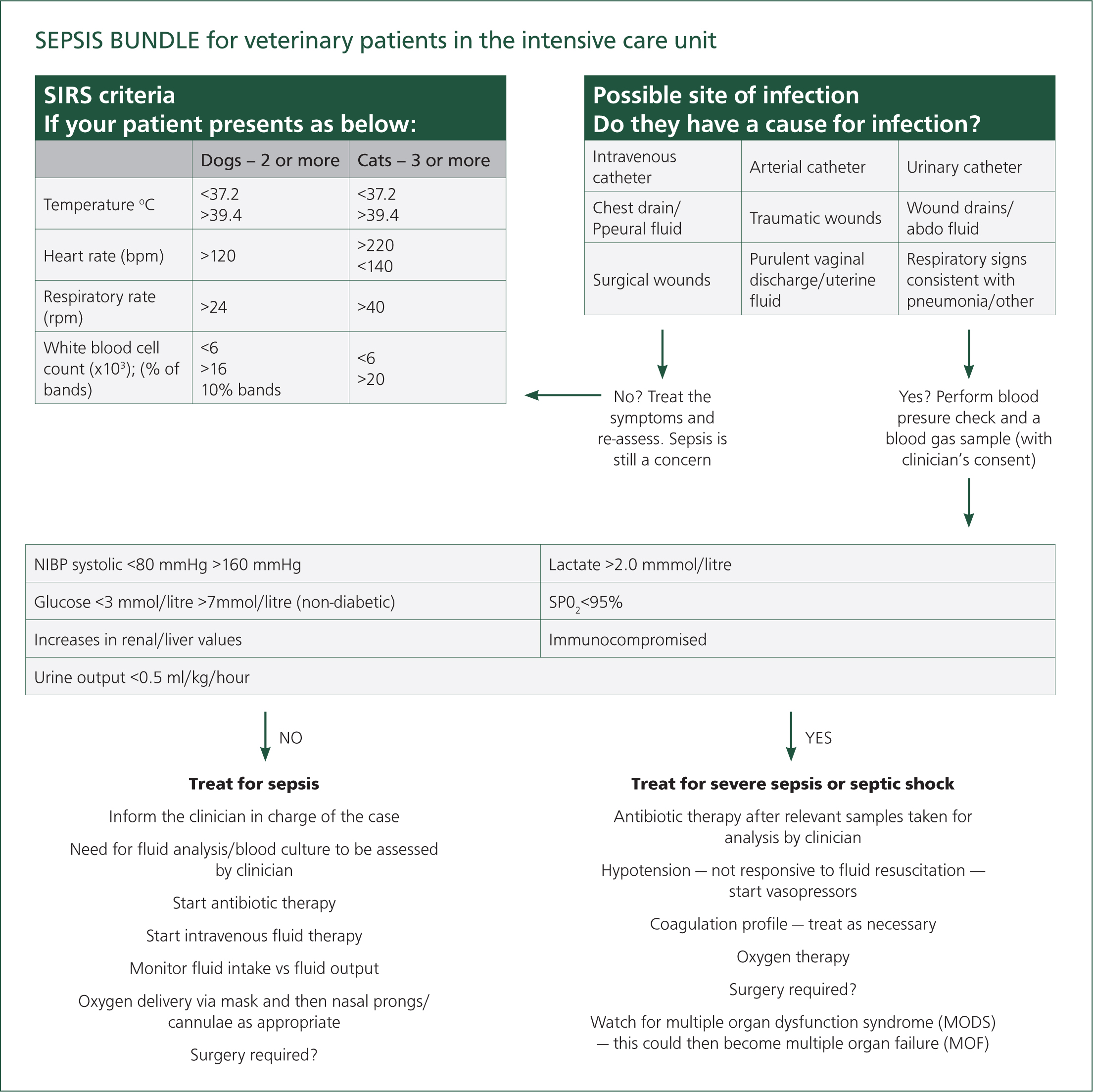
Sepsis is a real concern in patients with MODS, and it is vital that the signs of sepsis are identified quickly and treated aggressively (Moore, 2016). RVNs typically spend more time with the patient than other members of the veterinary team, which puts them in the prime position to spot changes in a patient's clinical condition early. The author proposes that the introduction of a sepsis bundle in the hospital's ward area may help RVNs to recognise early signs of sepsis in their patients, thus allowing for earlier veterinary intervention. In human hospitals, sepsis bundles are widely implemented and have proven to significantly reduce sepsis mortalities amongst patients (Levy et al, 2018). A veterinary sepsis care bundle, produced by Gray (2017), was adapted using the clinical parameters from the Hauptman et al (1997) criteria. Gray, (2017), trialled the bundle in a busy intensive care unit (ICU), and although it was a small trial, it highlighted that a sepsis care bundle could raise awareness and potentially improve patient outcomes once sepsis is suspected and goal directed therapy is started early (Figure 4). Currently, there is no designated ICU in the hospital and critical patients are treated in the main ward. It may help to include a laminated version of this model in the main ward, which could be used in combination with a short care plan, as suggested by Gray (2017). This, along-side specific training, may increase knowledge of sepsis and provide better awareness when nursing critical patients, which could reduce morbidities and mortalities resulting from sepsis and MODS.
Additionally, this patient may have benefited from an indwelling urinary catheter and closed collection system to allow accurate quantification of urine production (Ross, 2011). Azotaemia, defined as an increase in urea and/or creatinine (Whittemore and Webb, 2005), was highlighted on this patient's blood results. This could have indicated the beginning of an AKI or pre-renal azotaemia associated with dehydration (Legrand and Payen, 2011). Normal urine output should be maintained at 1–2 ml/kg/hour, however if urine output remains lower than this, it could be an indicator that the patient is inadequately hydrated, or suffering from oliguric renal disease (Whittemore and Webb, 2005). AKI typically presents as oliguria, therefore, it is important to regularly monitor and record urine output and bodyweight in these patients, and alert the VS to any changes (Whitehead, 2014). The placement of a urinary catheter, however, is not without risk because of the possibility of ascending infection, and is at the discretion of the VS (Ross, 2011).
Conclusion
The patient was discharged after 6 days and went on to make a full recovery. Treatment of MODS remains largely supportive requiring exceptional teamwork and communication between members of the veterinary team. RVNs continue to be at the forefront of care, which highlights the need for appropriate monitoring. With a range of basic and advanced monitoring interventions available, RVNs are encouraged to provide holistic nursing care that is tailored to meet the needs of critically ill patients. Additionally, RVNs should gain insight into the underlying physiology of these disease processes through further training and self-learning, as it will help them to recognise changes in a patient's clinical status, thus allowing care to be adjusted promptly.
KEY POINTS
- Sepsis is a common inciting cause of multiple organ dysfunction syndrome in veterinary patients.
- Early detection of sepsis is crucial to increase the chances of survival.
- Basic monitoring of the patient's cardiovascular, respiratory and neurological systems are simple nursing tasks that can make a big difference in the early detection of sepsis.
- Having an understanding of the physiology behind the patient's vital signs can vastly improve patient outcomes. Veterinary nurses spend the most time with these patients and are therefore able to notice and act on signs quickly.
- The use of sepsis bundles has proven to increase survival rates in human medicine, and therefore, should be encouraged within veterinary medicine to decrease the rate that sepsis progresses to multiple organ dysfunction syndrome.


