The majority of wounds encountered in practice are either surgically created, or traumatic in origin. In order to deal with these wounds effectively, it is important that veterinary nurses understand the complex nature of wound healing, and the biological chemicals that are essential to normal wound healing. When dealing with open wounds, the aim is to enhance the wound healing process, so it can occur as quickly as possible, in a way that causes as little discomfort to the patient as possible, and in a cost-efficient manner for the client.
The local wound environment is hugely important as there needs to be a balance of biochemical products, e.g. chemotactic factors, eicosanoids, free radicals, cytokines, growth factors, inflammatory cells, and enzymes (Krahwinkel and Boothe, 2006). These products are important in wound healing because they break down the less desirable structures within a wound, e.g. via autolytic debridement, and also provide chemical signals and biological components required for wound healing.
The biology of wound healing is complex. Normal wound healing involves three key stages: inflammatory, proliferative and remodelling stages, with each phase exhibiting overlapping processes of coordinated cellular activities. Cytokines play a vital role in these cellular processes, enabling wound healing cells to produce the necessary structural proteins and polymers required for wound healing (Slavin, 1996).
One extracellular polysaccharide involved in wound healing is the glycosaminoglycan (GAG) hyaluronan. Hyaluronan is found throughout the body, in tissues and body fluids, and demonstrates some unique physiochemical and biochemical properties (Fraser et al, 1997). Hyaluronan plays a role in every phase of wound healing and studies have been performed looking at its role in wound healing, from angiogenesis (Borgnoni et al, 1996) to the activity of fibroblasts (David-Raoudi et al, 2008).
Wound dressing options
While the majority of wounds that are encountered will heal without any issues, there are a number of topical, and systemic medications that can enhance the wound healing process, allowing the wound to heal in as short a timeframe as possible, and without complications. A thorough understanding of wound healing and wound medications is essential in order to select the correct product for an individual wound, at a specific stage of wound healing; no one product works best for every type of wound.
The common dressings and medications used to enhance wound healing work in various different ways, including: maintenance of a moist wound healing environment (e.g. hydrogels, hydrocolloids); reducing oedema via hydrophilic action (e.g. Manuka honey); providing a source of healing substance (e.g. collagen, bioscaffolds); controlling infection (e.g. topical antimicrobials); and aiding debridement (e.g. Manuka honey, topical enzymes).
Hydrogels
Hydrogels are composed of 80–90% water, and are most commonly available as amorphous gels or impregnated gauzes. Hydrogels do have the capacity to absorb a very small amount of fluid, i.e. wound exudate, but can donate large amounts of moisture to the wound (Myers, 2004). Hydrogels promote a moist wound environment, and because of their ability to donate moisture and rehydrate tissue they are beneficial in dry wounds (Campbell, 2006). Hydrogels can be used for autolytic debridement and so are useful in necrotic wounds, and wounds with eschars, as they rehydrate the tissue, easing its removal (Eaglstein, 2001). The use of hydrogels has been studied in canine patients, with the use of hydrogels associated with increased wound contraction on limb wounds (Morgan et al, 1994), but delayed contraction on trunk wounds (Ramsey et al, 1995). Hydrogels are applied directly to the wound site, and then must be covered with a dressing in order to prevent dehydration; the hydrogel should be applied with care as if it is applied to the surrounding skin, maceration can occur (Campbell, 2006). Hydrogels are used in the later stages of wound healing, on wounds that have healthy granulation tissue, decreased drainage, and evidence of epithelialisation (Krahwinkel and Boothe, 2006).
Hydrocolloids
Hydrocolloids consist of compounds of pectin, gelatin, and carboxymethylcellulose with some type of film or adhesive backing (Campbell, 2006). Hydrocolloids have considerable absorptive abilities of between 75% and 650% of their weight in fluid (Myers, 2004). On absorbing wound fluid and exudate, the hydrocolloids swell into a gel-like mass, maintaining a moist environment conducive to healing (Krahwinkel and Boothe, 2006). Hydrocolloids are indicated for partial and full-thickness wounds and can safely be used on granulating and necrotic wounds (Myers, 2004). Wounds covered with hydrocolloids have lower infection rates in people than those covered with gauze, film, hydrogels, or foams (Myers, 2004). They also help to protect the wound from contamination and aid in autolytic debridement. These products are designed to remain on the wound for up to 7 days, but should be changed whenever strike-through occurs. People who are unfamiliar with hydrocolloids may be concerned by the appearance and odour of the dissolving residue. The purulent appearance and odour may give the false impression of infection; however, when the residue is removed by gentle lavage, the wound appears healthy. Excessive granulation can occur if hydrocolloids are used for too long, resulting in a proud granulation bed.
Alginates
Alginates are polysaccharide dressings produced from kelp (seaweed). They are available as twisted fibres or mats and are applied to wounds as a primary dressing. Sodium from the wound exudate and calcium from the alginate undergo ion exchange, resulting in a soluble alginate gel (Cho and Yo, 1998). A secondary dressing should be used to secure the alginate in situ. The gel produces a non-adherent moist wound environment that promotes autolytic debridement and wound healing. Alginate products are best used to treat moderate to heavily exuding wounds in the early stages of healing (Swaim and Gillette, 1998). If used on a dry wound, the alginate can be moistened with saline to avoid desiccating the wound or shedding fibres into the wound bed, and to avoid a wet-to-dry bandage effect on removal. If desiccation does not occur, the dressing can be left in place for up to 7 days or changed when there is strike-through of the secondary dressing (Cho and Yo, 1998). On removal, the alginate gel can be gently lavaged away without damage to the underlying tissue. The gel that forms on the wound may emit a foul odour and have a purulent appearance that can be mistaken for wound infection, but staff should be informed that this is a normal occurrence.
Polyurethane foam dressings
Foam dressings are composed of polymers (usually polyurethane), and are available in sheets of variable thicknesses. Foam dressings are highly absorptive and are designed for wounds with moderate to high levels of exudate. These dressings have a wicking action which draws exudate into the foam core and this feature is useful in removing moisture from periwound skin to avoid maceration due to excessive moisture left by less absorptive dressings (Campton-Johnson and Wilson, 2001).
Foams support moist wound healing either by absorbing excessive exudate or, if pre-moistened, by delivering fluid to the wound bed (Swaim and Gillette, 1998). Foam dressings promote epithelialisation, so they are a good choice after a granulation bed has formed and epithelial coverage is needed (Hendrickson, 2002).
After being cut to the shape of the wound, foam dressings are placed, without the need to premoisten, on exudative wounds, or pre-moistened with sterile saline or liquid medication before placement on dry wounds. They are changed every 3 to 7 days, or when strike-through comes within 1 inch of the foam edge, whichever is sooner (Seaman, 2002). Foams should not be allowed to dry out because they can then become incorporated into the granulation bed, making their removal difficult and traumatic (Kannon and Garrett, 1995).
Honey
The use of honey to treat wounds dates back to 2000 BC (Forrest, 1982). Honey has been used for cleansing and accelerating the healing of wounds for centuries; however, the scientific basis for its success was not determined until the 20th century. The mechanisms associated with wound cleansing and healing properties of honey include decreased inflammatory oedema, attraction of macrophages to further cleanse the wound, accelerated sloughing of devitalised tissue, provision of a local cellular energy source, and formation of a protective layer of protein over the wound and a healthy granulation bed (Kamat, 1993). Honey also has a deodorising action; this may be due to its rich supply of glucose, which would be used as an energy source by the infecting bacteria in preference to amino acids, resulting in the production of lactic acid instead of malodorous compounds (White, 1966). Honey also has antibacterial properties that have been attributed to its high osmolarity, acidity, and hydrogen peroxide (H2O2) content (White, 1966). Honey is reported to be effective against a wide variety of Gram-positive and Gram-negative organisms, including Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, and Staphylococcus aureus, including multidrug resistant strains (Krahwinkel and Boothe, 2006).
The role of hyaluronan in wound healing
As mentioned previously, hyaluronan plays a vital role in wound healing. As a molecule, hyaluronan is a negatively charged, disaccharide polymer and is found in variable lengths, depending on its location within the body (Frenkel, 2014), with each molecular size uniquely functioning at each wound healing phase. Large, heavy and long hyaluronan chains are thought to have structural function during the inflammatory phase, and also act as a space-filler in granulation tissue (Frenkel, 2012), whereas the small, light and short hyaluronan fragments have a stimulatory effect, and attracting properties, encouraging fibroblast migration, and collagen production (Moseley et al, 2002).
Hyaluronan has recently been incorporated into a wound spray, Remend™ Wound Spray Gel which has been developed to enhance wound healing, and to promote rapid wound closure in horses, dogs and cats. Remend™ promotes rapid wound closure as it provides a matrix to enhance the cellular events that are necessary for normal wound healing to occur; this is achieved due to the action of the cross-linked hyaluronan contained within the spray.
Hyaluronan has a hygroscopic nature, thereby providing an open, hydrated matrix, which facilitates the migration of cells into the wound bed. It also interacts with cells such as fibroblasts and keratinocytes, which enhances cell migration and proliferation, encouraging rapid wound healing.
Hyaluronan and the phases of wound healing
Hyaluronan plays a role in healing in a wide variety of wounds because the polymers exist in varying lengths. The individual molecule size uniquely functions on each wound healing phase, with the large hyaluronan molecules acting as space-filling molecules with regulatory and structural functions, whereas small hyaluronan fragments are involved in angiogenesis, inflammation and immunostimulation (Stern et al, 2006).
During the inflammatory phase of wound healing hyaluronan plays several roles:
- The hyaluronan fragments are synthesised from platelets and available hyaluronan in the bloodstream, and these fragments have the ability to bind to fibrinogen to initiate the extrinsic clotting pathway (Stern et al, 2006).
- Due to the release of hyaluronan following injury, the tissues within, and surrounding the wound, become slightly oedematous. This oedema causes swelling of the tissues surrounding the wound, which creates a framework for cells to migrate into the wound environment and the chemotaxis of inflammatory cells. This inflammatory process is driven by cytokines, which in turn result in blood vessel dilation, allowing increased cellular recruitment into the wound (Hart, 2002). This inflammatory process is essential as part of the wound healing process.
- Conversely, hyaluronan also has a role in reducing and moderating the inflammatory response (Wisniewski and Vilcek, 1997). Heavy hyaluronan chains prevent inflammation by inhibiting neutrophil migration (Noble et al, 2011) and inhibition of plasmin through negative feedback loops (Wisniewski and Vilcek, 1997).
Moving on to the proliferative phase, this stage marks the arrival of fibroblast migration (Halloran and Slavin, 2002). Fibroblasts create collagen and glycosaminoglycans (including hyaluronan), constructing and anchoring the newly formed extracellular matrix (ECM) (Enoch and Leaper, 2005). Hyaluronan has also been proven to ‘fill in gaps’ in the ECM (the early granulation tissue) (Chen and Abetangelo, 1999). Hyaluronan is also responsible for stimulating the matrix metalloproteinases (MMPs) that are essential for new capillary formation, by breaking down the basement membrane of the wound; this allows for new capillary buds to sprout from existing ones (Pardue, 2008). Kaya et al (1997) also noted how hyaluronan is required for effective epithelialisation, as without its signalling and physiochemical properties, excessive scarring and delayed wound healing may occur.
Finally hyaluronan is also involved in the remodeling phase. As wound strength increases progressively over time, the wound specific cells and structures and hyaluronan are gradually degraded and replaced by an avascular, collagenous scar (Enoch and Leaper, 2005).
These studies indicate that the use of hyaluronan is beneficial to wound healing during the inflammatory and proliferative stages, as its presence has a very specific purpose in the wound healing process.
Case Study — Leo Forshaw
Leo presented following a road traffic accident during which a severe shearing injury to the medial aspect right carpus was sustained (Figure 1), with instability at all levels of carpal joints, and significant loss of styloid process and radial carpal bone. Open wound management was performed for 3 days prior to surgical repair when a modified type 2 external fixator was placed along with cross pinning of pancarpal joint to achieve an arthrodesis of the joint (Figure 2).
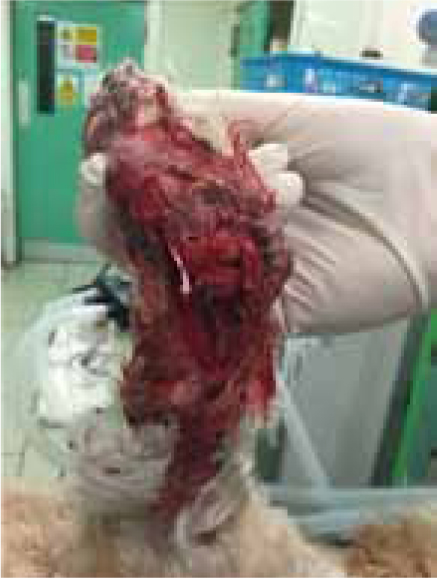
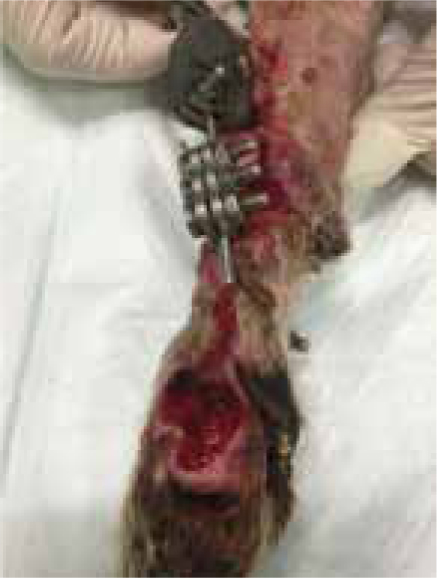
At surgery it was noted that approximately one third of the radial carpal bone was missing as a result of the injury, along with a large area of the radius medially, and all collateral support at every level.
The severity of the shearing injury meant that all wounds were not suitable for closure, due to the extent of the tissue loss, and required a period of open wound management. The wounds were dressed using Remend™ (Figure 3) in combination with an antimicrobial foam dressing (due to the level of contamination of the surgical site). Initially dressing changes were performed every 4 days, but by day 21 the dressing interval was increased to 7 days.
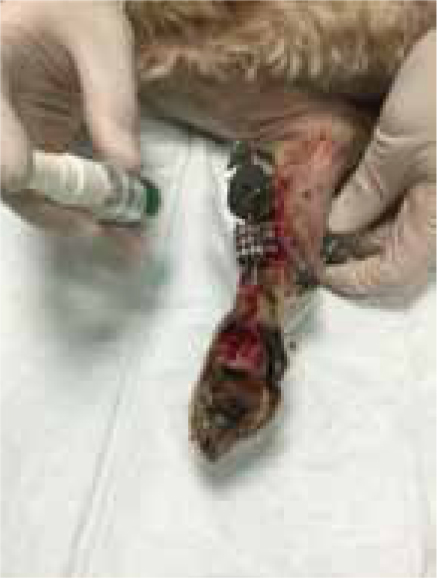
Remend™ and foam dressings continued to be used throughout the period of open wound management and the wound progressed well (Figure 4), with dressings no longer being required 7 weeks post injury (Figure 5).
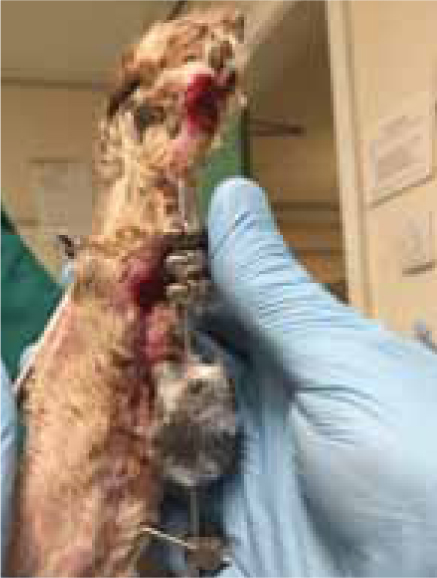

Case Study — Izzy Dixon
Izzy was referred to the hospital by her own veterinary practice following a road traffic accident. She had a suspected fracture of the right forelimb along with the presence of a pneumothorax and pulmonary contusions. Izzy was not stable enough to permit general anaesthesia and full surgical exploration of the wounds for 4 days following the trauma. On day 4 general anaesthesia was performed to allow radiography of the forelimb and wound assessment. Izzy was found to have a comminuted fracture of the right radius along with a severe, full-thickness abrasion wound down the medial aspect of the entire right forelimb, which would require a prolonged period of open wound management, with the possibility of a free skin graft. At surgery a modified type 2 external fixator was placed, which resulted in good axial alignment and reduction of the fracture site. The open wounds were dressed using Remend™ in combination with an antimicrobial foam dressing. Two days later the wound was redressed, but at this point there was some sloughing of the tissues over the medial aspect humerus and antebrachium, so the wound was redressed using a combination of manuka honey and a foam dressing to permit autolytic debridement of any remaining necrotic tissue (Figure 6). A swab was also taken for culture and sensitivity, which revealed the growth of Proteus spp. and Coliform spp. so marbofloxacin was added to the patients antibiotic protocol along with the existing cephalexin. Four days later the wound was redressed and any remaining areas of necrotic tissue were surgically debrided; the wound was redressed with Remend™ and an antimicrobial foam. Due to the extent of the tissue loss a prolonged period of open wound management was required, and the combination of Remend™ and foam dressings were used throughout this period, the wound progressed well and by 5 weeks post injury the wound over the antebrachium could be partially closed, due to the extent of the contraction and re-epithelialisation of the wound (Figure 7a and b). All remaining wounds continued to be treated using Remend™ and foam dressing. The wounds progressed well with all wounds being fully healed 2 months post injury (Figure 8), but the external fixator remained in place.
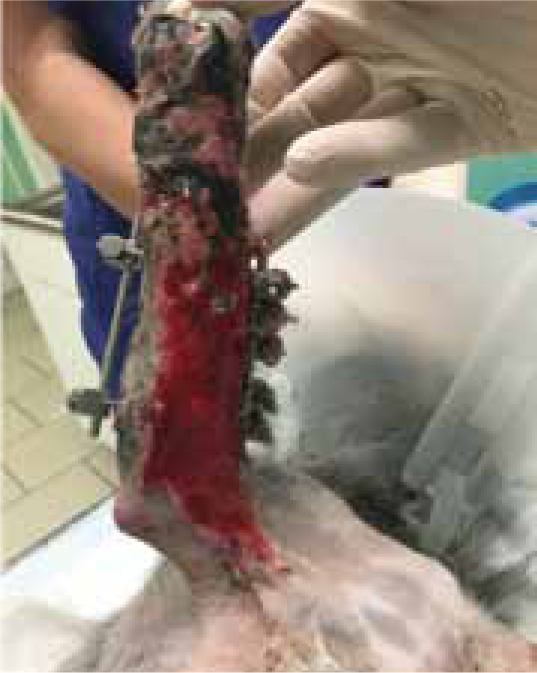
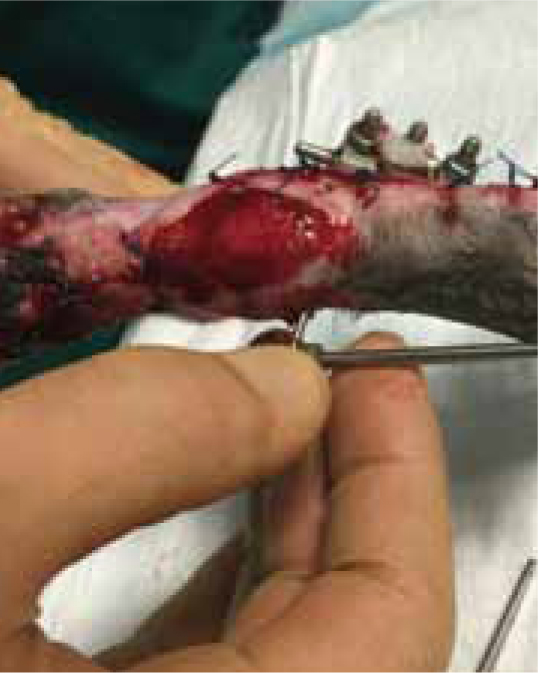
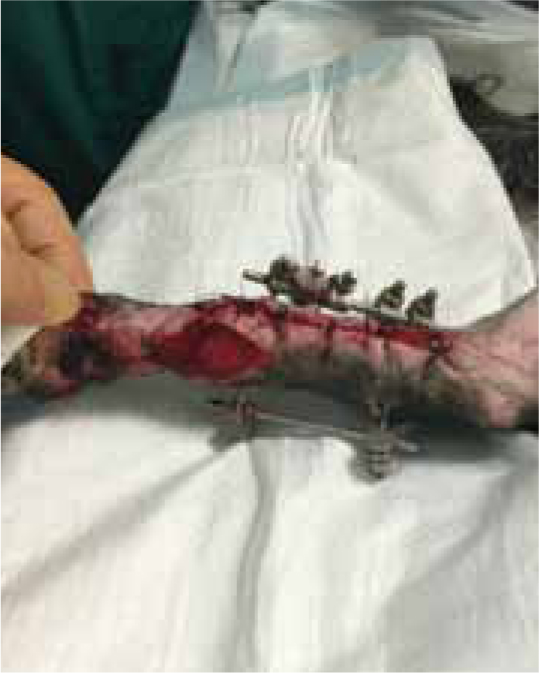
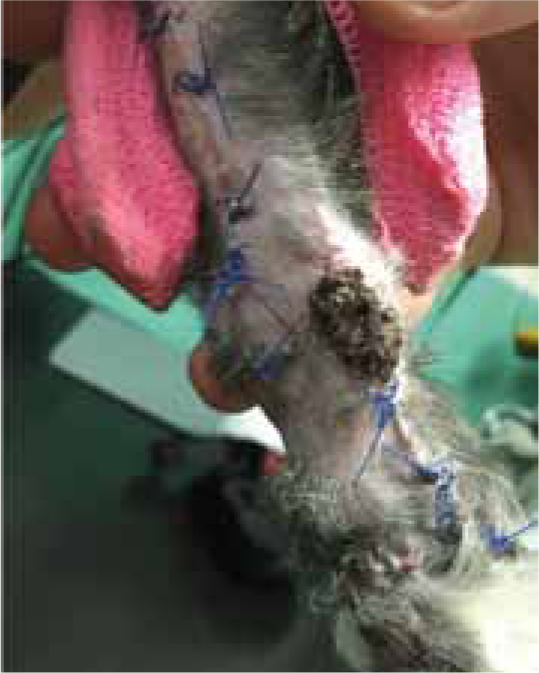
Case Study — Max Gavin
Max presented following trauma to his left hindlimb and was found to have a dislocation of metatarsal 4/5, and fracture to the 2nd and 3rd metatarsal bones, the fracture was open, with wounds present on the cranial and lateral aspect of the limb. Surgery was performed 2 days later (21/07/2015), but the primary surgical wound was difficult to close, without excessive tension. The decision was made to create tension relieving incisions, which resulted in numerous small wounds (created by a number 15 blade), but allowed the closure of the wound over the bone plate, without excess tension (Figure 9a and b). The wound was dressed using Remend™ and an antimicrobial foam dressing and revisited 3 days later.
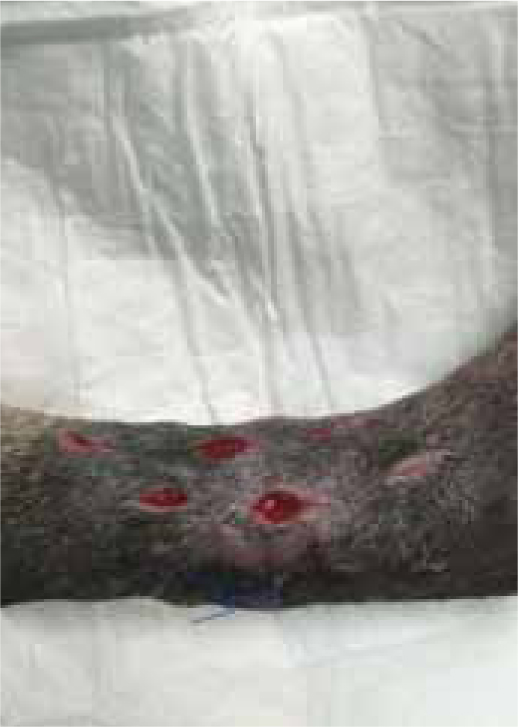
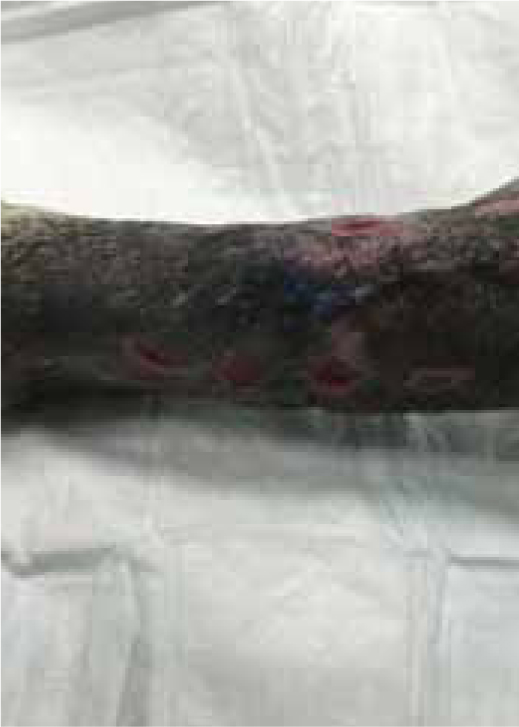
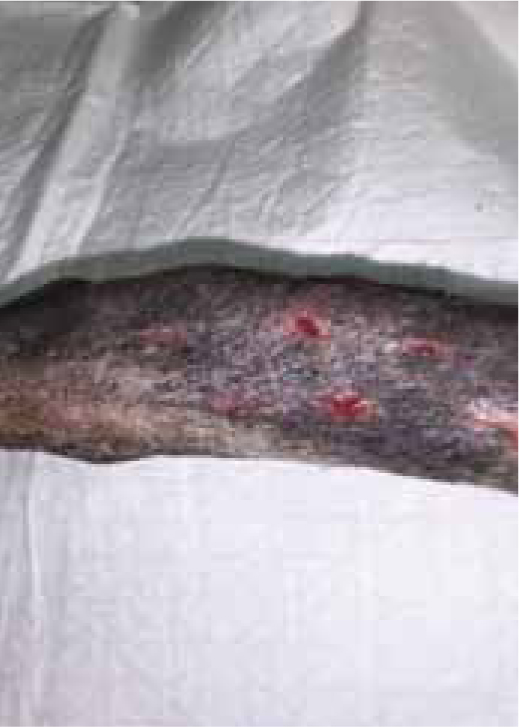
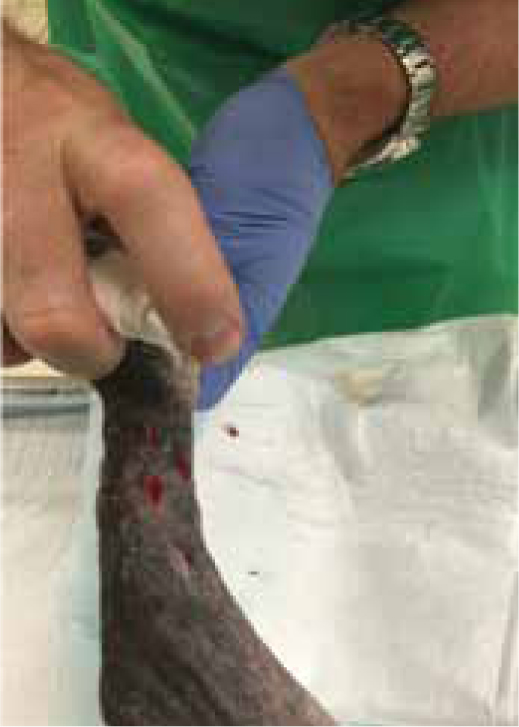
Figures 9b and 9c show the wound had made good progress, with no evidence of infection or dehiscence of the surgical site. The wounds were redressed again using Remend™ and the antimicrobial foam dressing — during the redress the wounds were not cleaned using any type of solution since there was no excessive amount of exudate present, and since this was contraindicated according to the manufacturer's instructions (Bayer Plc, Animal Health Division). The Remend spray was applied directly onto the wounds, and then covered with a foam dressing (Figure 10). The wounds were redressed every 4 days before the decision was made that the reapplication of Remend™ spray was no longer required (20 days from the original date of surgery) (Figure 9c). The limb did remain dressed due to the extensive underlying orthopaedic injuries.
Conclusion
The numerous ways in which hyaluronan plays a role in wound healing, means that RemendTM is highly beneficial to open wound healing as an addition to moist wound healing protocols. Its use should be considered early on in the wound healing processes as it contributes to the inflammatory stage by facilitating the migration of cells into the wound bed, and also interacts with specific cells during the wound healing process, e.g. fibroblasts and keratinocytes, to enhance cell migration and proliferation, thereby encouraging rapid wound healing. RemendTM is also useful in the granulation process as it has a stimulatory effect on MMPs, thereby encouraging new capillary formation. Overall RemendTM should be used as an addition to a wound healing protocol, and potentially could be useful in slow to heal wounds.
Key Points
- The local wound environment is hugely important as there needs to be a balance of biochemical products.
- Normal wound healing involves three key stages: inflammatory, proliferative and remodelling stages, with each phase exhibiting overlapping processes of coordinated cellular activities.
- Hyaluronan is found throughout the body, in tissues and body fluids, and demonstrates some unique physiochemical and biochemical properties.
- Hyaluronan polymers exist in varying lengths, with each molecular size uniquely functioning on each wound healing phase.
- Remend™ promotes rapid wound closure as it provides a cellular matrix to enhance the cellular events that are necessary for normal wound healing to occur.
- Hyaluronan has a hygroscopic nature, thereby providing an open, hydrated matrix, which facilitates the migration of cells into the wound bed.


