Wound management and treatment can be a challenging and confusing subject. Advancements in reconstructive surgery, debridement techniques and the rise of evidence-based veterinary medicine have increased knowledge of the efficacy of a wide range of treatments. However, with a more natural approach to veterinary medicine being sought by clients, new products are constantly being produced and wound treatment has come under serious scrutiny. In a constantly changing field it can prove difficult to decide on the best course of treatment.
Throughout history honey has always played a role within medicine, notably in wound management; and over the last few decades the benefits of honey for wound management have been evidenced through clinical studies and extensive research into new treatments for prevailing antibiotic resistant bacteria. The osmotic nature of honey can provide a form of autolytic debridement, along with anti-inflammatory properties and broad-spectrum antimicrobial activity, which has been demonstrated to be effective against a variety of organisms, including meticillin-resistant Staphylococcus aureus, Salmonella spp. and Pseudomonas spp. In addition to this, application of honey encourages a moist wound environment and an acidity level which has been shown to be beneficial for cellular activity in chronic non-healing wounds (Willix et al, 1992).
This article discusses the use of Manuka honey as a natural alternative to the clinical use of antibiotics and antiseptics for the treatment of necrotic, devitalised and infected wounds; along with supporting case studies, it will highlight the benefit of using manuka honey during the inflammatory phase of healing, and provide some considerations for its use.
A brief history of honey
The use of honey has featured throughout history with links to religion, cult and mythology, but probably one of the most prominent uses has been medicinally. The medicinal use of honey has been noted as far back as the Sumerian civilisation, and its use in antiseptic solutions has been documented in various prescriptions from this era (Teall, 2014). The Egyptians also demonstrated the use of honey in wound management, such as using bindings and poultices impregnated with honey to aid in the treatment of wounds (Bhattacharya, 2012).
Up until the late 1940s honey had been a widely used product, but there was a marked decline in its use when antiseptics, antibiotics and improvements in surgical procedures became more widespread (Gethin, 2008). From the 1980s the use of honey once again increased due to the development and introduction of sterilised devices containing honey (Cooper and Jenkins, 2009). The sterilisation process involves the filtering out of wax and other contaminants, followed by cold sterilisation using gamma radiation to in-hibit spore growth without denaturing the antimicrobial effects of the honey (Hollis, 2012). Today, there are numerous medical grade honey products available to both human and veterinary practitioners.
Pitfalls
With the use of honey on the rise, and more successful studies being published on the benefits of new honey-based wound preparations, media and public interest has boomed. This has led to the purchase and application of non-sterile honeys, potentially containing Clostridial botulinum (which has the potential to cause wound botulism) and other organisms, being used for wound management. A comparison study in 2009 looked at the antimicrobial efficacy of medical grade honey in relation to table honey (Cooper and Jenkins, 2009). This study looked at the anti-microbial properties and microbial flora of 18 table honeys and compared this with a medical grade manuka honey, finding that the table honeys exhibited relatively low antimicrobial activity compared with the medical grade manuka honey. The presence of potentially pathogenic organisms was also detected, and these may be harmful to already high-risk patients, although the study's authors noted that to date there had not been any reported infections traced to the use of non-sterile honey. It would be negligent to ignore the potential risks, so when advising use of honey as professionals we should consider the limitations of store-bought honey for use in wound management in a clinical setting (Copper and Jenkins, 2009).
Antimicrobial mode of action and manuka honey
Honey is a supersaturated composition, mostly comprising sugars with a low water content of approximately 17%. The colour, viscosity, smell and acidity level of honey is dependant on the region, floral source and foraging bee. Most honeys exhibit a pH in the range of 3.4–6.1, making it an acidic solution (Van der berg et al, 2008). The pH level is what prevents the facilitation of bacterial growth as most bacteria are pH sensitive and can only reproduce in certain pH ranges. Darker honeys tend to have a higher anti-oxidant content and are usually produced in the Southern hemisphere, predominantly in New Zealand. The most well known example is honey from the Leptospermum spp. (the manuka tree), or as its more commonly known, manuka honey.
Research discussing the antimicrobial effects of manuka honey began in the mid 1980s. Professor Molan noted the unusual activity of manuka when it was tested against a variety of bacterial spores, however it was not until later that the mechanism of action for manuka honey was fully understood (Carter et al, 2016). The antibacterial process of most honeys is caused by slow dilution of honey by enzymes in the wound bed, although it should be noted that this process is not exhibited equally by all honeys. The primary result of this dilution process is the activation of glucose oxidase, which forms gluconic acid and hydrogen peroxide (Airborne honey, 2018). Despite exhibiting fair antimicrobial properties, hydrogen peroxide can at high concentrations cause cellular and protein damage, in turn creating oxygen radicals which inhibits wound healing and can cause maceration of healthy surrounding tissue (Bang et al, 2003). Further to this, it is thought that the antimicrobial process of hydrogen peroxide may be slowed by increased temperatures and by wound exudate containing catalase, which causes the hydrogen peroxide to degrade (Cooper and Jenkins, 2009).
Manuka honey, however, behaves differently to other honeys. Known as a non-peroxide activity (NPA) honey, even though it does still produce low levels of hydrogen peroxide, the antimicrobial effect remains even after this is neutralised (Hollis, 2012). This is believed to be due to a naturally occurring phytochemical, methylglyoxal, which reacts with relatively non-specific macromolecules, and although there is potential for this to be toxic to mammalian cells, this has not yet been exhibited when ingested or used in topical wound management. Why this process is selectively toxic to bacterial cells and not mammalian cells is unknown (Carter et al, 2016).
Research has demonstrated that the pH levels within a wound bed can interfere with the body's natural healing process. A more alkaline environment can reduce rates of healing when compared with those maintained at a more acidic level. Manuka honey's acidic properties, ranging between a pH of 3.2–4.5, significantly lower than some honeys, has been shown to be beneficial in chronic non-healing wounds (Nagoba et al, 2015). This acidifying effect in certain studies has shown to increase healing rates among human patients with chronic wounds (Rendl, 2001). This increase in healing was believed to be related to decreases in pH levels leading to a rise in oxygen release from haemoglobin in the capillaries (Simon et al, 2009); although, more recent research has shown that lower pH levels cause a suppression of proteases in the wound bed (Simon et al, 2009). Proteases destroy proteins and growth factors, this means that an excessive amount of protease at the wound site can cause the breakdown of protein fibres and the fibrin matrix — meaning fibroblasts and epithelial cells will struggle to migrate across the wound bed, leading to a prolonged inflammatory phase (Schultz et al, 2005).
Manuka honey is graded for its antimicrobial effect using a grading system known as the unique antimicrobial manuka factor (UMF®). This grading system represents the purity and quality of the manuka honey produced (Hollis, 2012). The higher the number, the more intense the antimicrobial effect, and for use in wound management a minimum of +10 should be used (Table 1)(Molan, 2012).
| UMF® | Potential use |
|---|---|
| 0–4 | Not detectable — not able to display UMF® logo |
| 5–9 | Use as a supplement for general wellbeing |
| 10–14 | Suitable for therapeutic use |
| 15 and higher | Superior levels with high levels of activity for therapeutic use |
Manuka honey and antimicrobial resistance
Antimicrobial resistance is of increasing concern for both the human and veterinary medical professions. The overuse of antibiotics and the lack of development of new and novel therapies to combat the issue has caused this emerging problem to hit a critical point. One solution to this increasing problem has been the investigation and introduction of natural therapies as an alternative to the more conventional use of antibiotics (Roberts et al, 2015).
Manuka honey has been tested in-vitro against a variety of bacterial species, in particular pathogens that tend to colonise on the skin surface and mucosal membranes. A review of manuka honey collated a wide range of data produced by these in-vitro studies. The authors found that to date, when tested against multidrug resistant bacteria, there was no reduction in their sensitivity, showing that manuka honey exhibits a broad-spectrum antimicrobial activity un-like any other known antimicrobials (Tan et al, 2009). In addition, attempts have been made to create honey-resistant bacteria in a laboratory setting, which so far have been unsuccessful (Blair et al, 2009; Cooper et al, 2010).
Human medical research into combined therapies has found that sub-inhibitory concentrations of manuka honey can be used concurrently with conventional antibiotic therapies. This combination therapy showed a reduction in the minimum inhibitory concentration of the antibiotics used, effectively ‘reversing’ antimicrobial resistance (Jenkins and Cooper, 2012). As such evidence is increasingly supportive of the efficacy of manuka honey as both a combination therapy, and an alternative standalone product, in place of traditional antibiotics. However, research is still considered to be limited and further investigation is required to make the use of manuka honey more consistent within the clinical environment (Roberts et al, 2015).
Firefighting: the inflammatory phase
The second stage of wound healing is the inflammatory phase. This begins immediately following injury and usually lasts up to 3 days (Shipperley and Martin, 2002). After an injury occurs the body kickstarts an emergency response by means of vasoconstriction of the blood vessels preventing further haemorrhage. This results in blood clot formation, and if this process does not occur then healing cannot begin (Brown, 2015). Once haemorrhage is controlled, the blood vessels within the wound dilate and release transudate fluid which causes localised swelling (Figure 1). This release of transudate fluid allows antibodies, white bloods cells, growth factors, essential enzymes and nutrients to enter the wound. This is a natural process and only becomes problematic if it is prolonged or excessive (Clinimed, 2018). This leads to a rise in wound exudate predominantly containing phagocytic cells, which start to autolyse devitalised and necrotic tissue (Figure 2). Manuka honey's debridement properties make it useful during this stage of healing, and its anti-inflammatory effect can help by reducing swelling, oedema and redness. The mode of action for this process is not fully understood, but various clinical trials have demonstrated anti-inflammatory effects when used on burns, gingivitis and non-healing leg ulcers in humans (Molan and Rhodes, 2015).
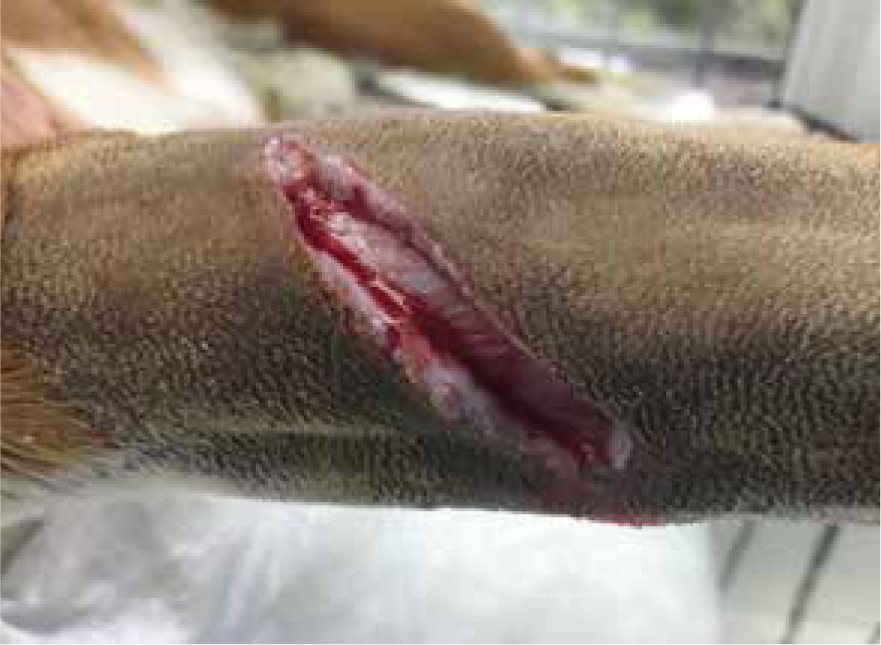
Autolytic debridement and manuka honey's role
Debridement is the process by which foreign material, bioburden or devitalised tissue is removed to promote healing or expose healthy tissue (Peters, 2013). There are several mechanisms of wound debridement, for example, autolytic, mechanical, sharp, and bio surgical. A brief description, along with advantages and disadvantages, are summarised in Table 2. This article will mostly look at the benefits of autolytic debridement and how manuka honey can aid in this process.
| Debridement method | Advantages | Disadvantages |
|---|---|---|
| Autolytic: lysis of damaged tissue at the wound site, performed naturally by the body's inflammatory process and can be encouraged with use of hydrogels, alginates, hydrocolloids and honey |
|
|
| Mechanical: uses force in the form of wet-to-dry dressings, high-pressure irrigation, pulsation therapy and wound ‘scrubbing’ to remove devitalised tissue via a non-selective process |
|
|
| Sharp: use of surgical instruments to selectively remove devitalised tissue |
|
|
| Bio surgical: form of maggot or larval therapy |
|
|
| Chemical or enzymatic: uses chemicals applied to the wound bed to selectively breakdown necrotic tissue and biofilms. Common preparations are ointments containing trypsin, proteinase and collagenase (Murgia, 2016). |
|
|
When a traumatic injury occurs, the body will begin its own natural process of autolytic debridement (Figure 3). This process refers to the lysis, or breakdown, of damaged tissue at a wound site. This inflammatory response will release enzymes that will digest proteins, fibrin and collagen (Brown, 2013). This ‘natural’ autolysis is performed by the body every time an injury occurs. As such, when looking at managing a wound we are not necessarily accelerating or changing this natural process with the addition of wound products or the use of different debridement techniques, but rather creating an optimal environment to help aid it through its natural process and begin the next phase of wound healing.
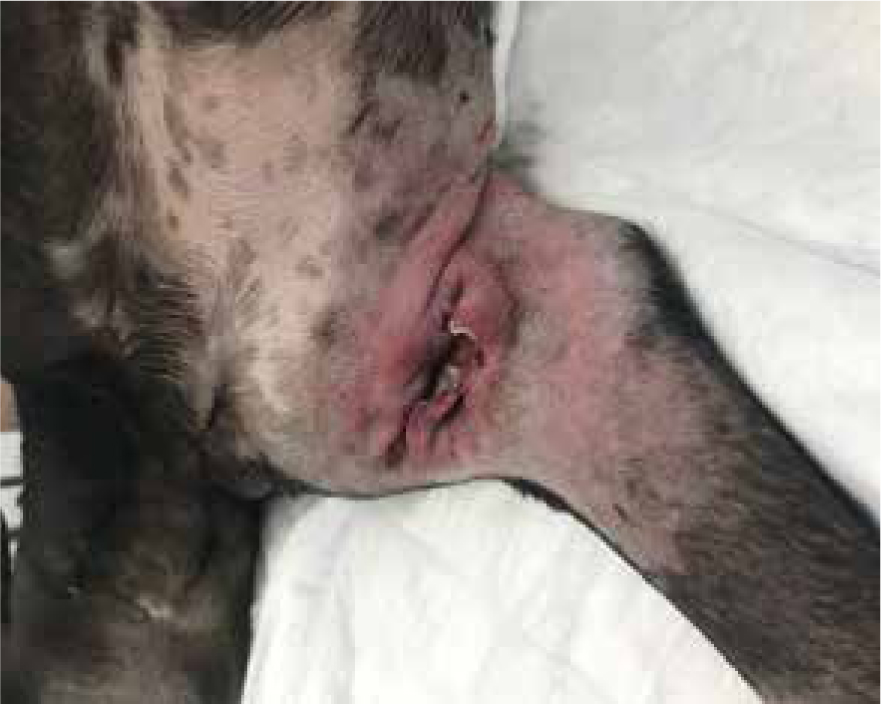
The debridement action of honey is highly effective. Not only does its low water and high sugar content create an osmotic potential, but the release of low levels of peroxide works concurrently to create an optimal environment for the body to carry out its natural debridement process (Hollis, 2010). The osmotic process causes lymph to rise from deeper wound compartments to the wound surface, helping to cleanse the wound, and low levels of peroxide production aid in the breakdown of dead cells, debris and fibrin, while both these processes reduce bacterial burden by removing excess water needed to make proliferation possible (Gethin, 2008). This leads to the stimulation of natural proteases derived from neutrophils to aid in the digestion of proteins (Hollis, 2012). As such, manuka honey is a good choice for use during the inflammatory phase of healing, supporting the natural healing process from the inflammation phase into the proliferation, then maturation phases (Figure 4).
Considerations
Research by Winter(1962) demonstrated that maintaining a moist wound environment can optimise healing, however it is important to use the right product for the right phase of healing. Manuka honey aids the body in continuing to breakdown devitalised and necrotic tissue, but care is needed when applying manuka honey to wounds as the peroxide process can macerate healthy tissue and cause exudate levels to rise (Hollis, 2012). Therefore, the secondary dressing needs to exhibit suitable absorbent properties (Figure 5 and 6) for the levels of exudate being produced. Barrier creams can be applied around the wound to help prevent maceration of healthy surrounding tissue (Figure 7).
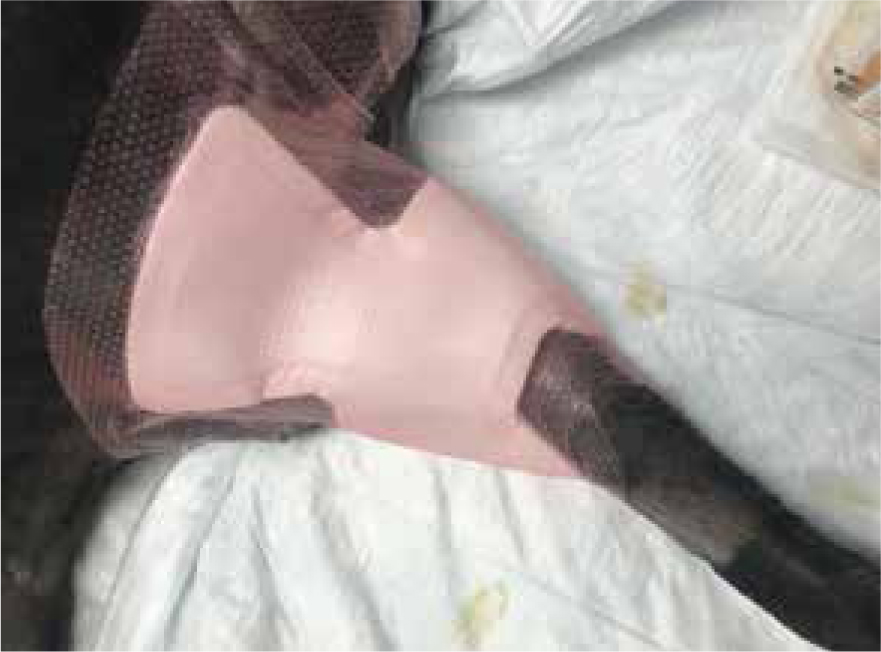

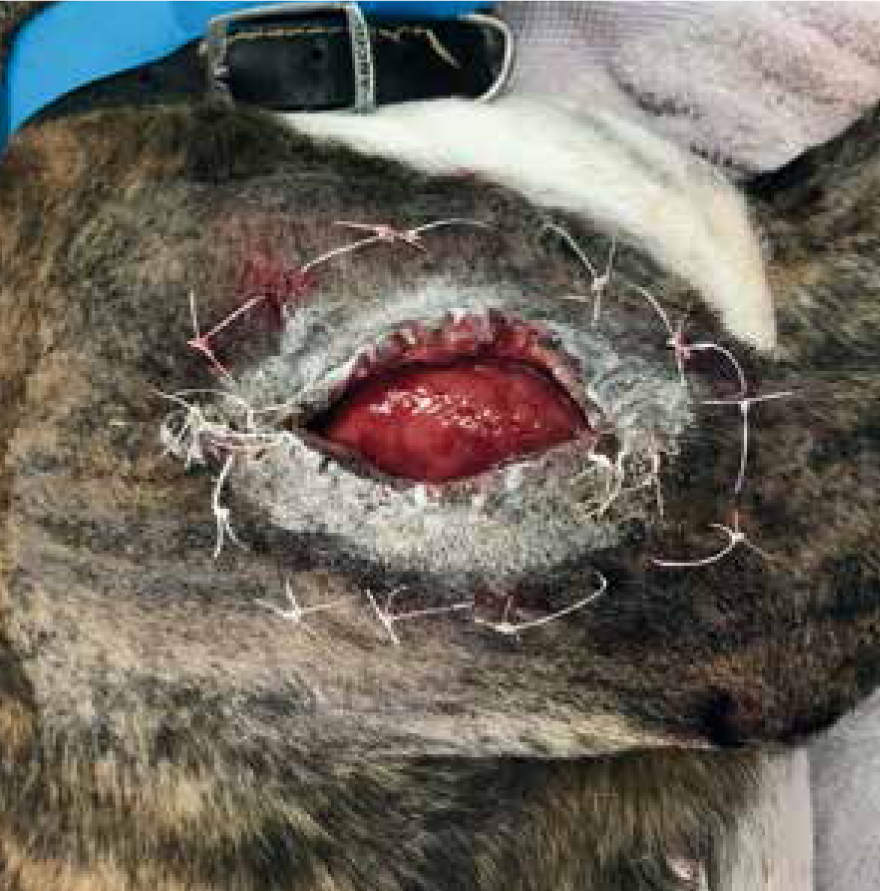
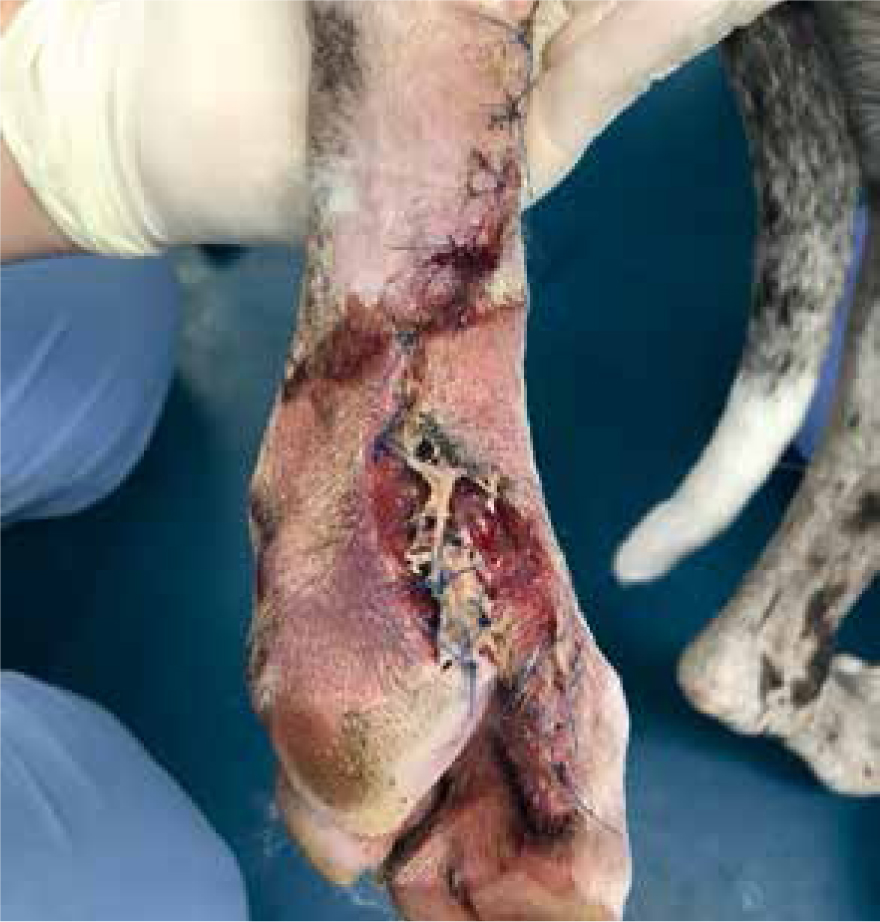
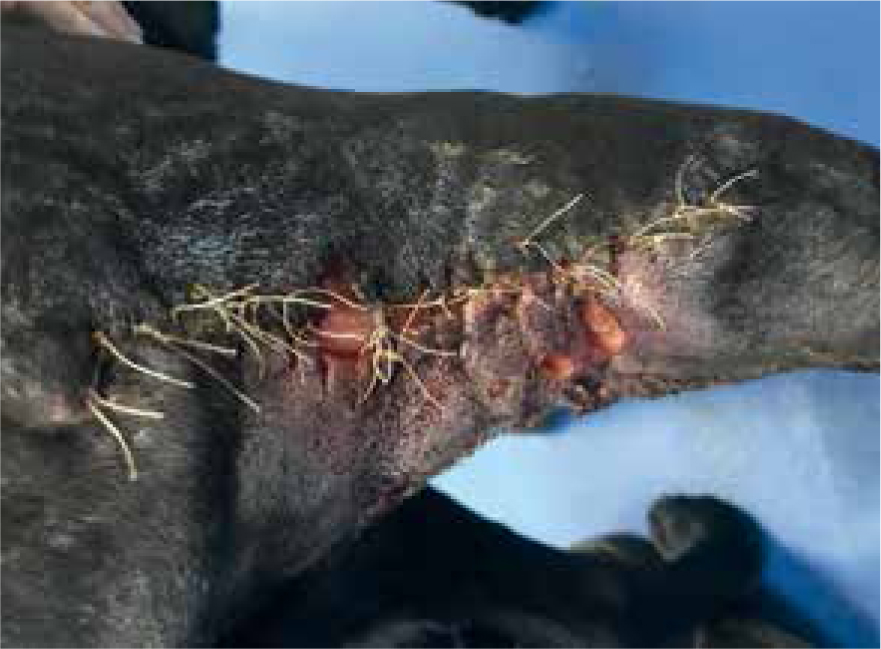
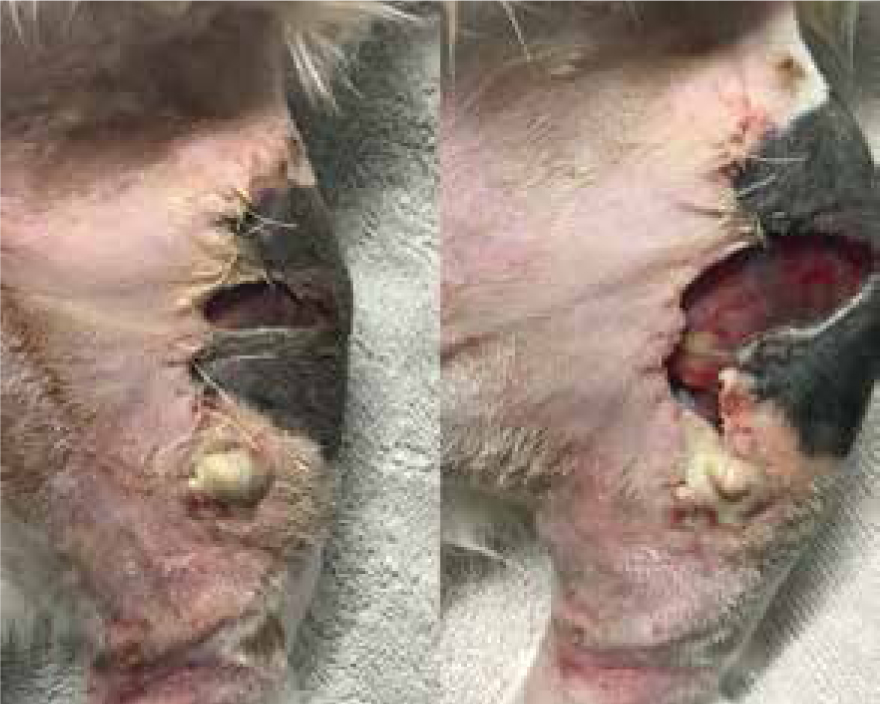
Patients with previous anaphylactic reactions to bee venom or bee products should be carefully evaluated before use (Hollis, 2017). Manuka honey's osmotic effects may cause complications with arterial bleeds or wounds that are actively haemorrhaging, as it may encourage further bleeding (Hollis, 2017). When considering its use for post surgical wound dehiscence, if there are sutures remaining, especially dissolvable sutures, these may breakdown more rapidly in the presence of honey. Therefore, a clinical decision should be made by the veterinary surgeon on whether these sutures are still required or whether they are just acting as foreign material (Figures 8 and 9) (Hollis, 2017). The natural properties of honey, low pH and high sugar content, can hinder fibroblast activity when used during the proliferation stage of healing, which can exacerbate the growth of granulation tissue (Hollis, 2017).
Applications and practical use
Wound care companies have continued to create more unique ways to use manuka honey in wound management. Medical grade manuka honey can now be found in a variety of preparations that are suitable for different types of wounds, these include the standard sterilised tubes of manuka honey, thicker more viscous gel preparations, impregnated dressings and foam preparations (Table 3). Care should be taken when selecting products to consider the price and properties of the manuka honey being bought, as these can vary widely.
| Product type | Use | Product views | Product examples |
|---|---|---|---|
| Tubes: most having a UMF® of 12 and above, sometimes available in a more viscous gel form |
|
|
|
| Manuka honey impregnated Tulle: a knitted cotton or viscose mesh dressing (NICE, 2018a) |
|
|
|
| Manuka honey impregnated alginate: non-woven fibrous, non-occlusive dressing usually made from calcium alginate or calcium sodium alginate (derivative of brown seaweed) which is then impregnated with manuka honey (NICE, 2018b) |
|
|
|
True medical grade manuka honey products, with a UMF of 10+, are tested by registered laboratories before being distributed. Licensed wound care products are subject to testing that ensures batches are produced consistently and manufactured according to specific guidelines provided by authoritative bodies (Cooper and Jenkins, 2009). Manuka honey produced for food consumption does not undergo such rigorous testing, and the pasteurisation process will not eliminate Clostridium botulinum spores. These spores are generally harmless if consumed, except in infants under 12 months of age (Cooper and Jenkins, 2009), but in wound management the dilution process means the spores could become viable and have the potential to cause wound botulism (Olaitan et al, 2007).
Inevitably the increase in the commercial use of manuka honey has caused demands for the product to rise, this unfortunately has resulted in food grade manuka honey being blended with other honeys sourced from nectar related to the manuka plant and being sold as manuka honey (Hollis 2012). The most common is from the Australian jelly bush, but it is not genuine manuka. So, if manuka honey is being recommended it should be made clear that store-bought products should be avoided as labelling can be misleading and inconsistent, and its use could cause adverse reactions in already potentially compromised patients (Hollis, 2012).
Example case studies
Case study 1: manuka honey and burns
Breed, gender and age:
Feline, domestic shorthair, neutered female, 15 years, body condition score (BCS) 2/5. Note, the cat was known for severely aggressive behaviour when hospitalised, so sedation was required for every dressing change.
Wound location and presentation:
Presented to practice after being trapped inside the owner's car engine when car started, presented with signs of shock, hindlimb paralysis and full thickness burns to caudal stifle and tail. Stifle wound tracking approximately 7 cm proximally into groin area, radiographs showed no signs of fractures or foreign material, but severe sub cutaneous emphysema. Tissue inflamed with marked maceration of the tissue noted — full extent of burns took a few days to fully manifest. This article will focus on the stifle injury.
Day 1
After stabilisation wounds were clipped, lavaged using warm isotonic solution and thoroughly examined to determine the full extent of the injuries (Figure 10). After wound preparation a manuka honey impregnated Tulle dressing was packed into the wound and manuka honey fill tube used on any areas that impregnated dressing did not reach. The decision to use manuka honey was made to try and help prevent infection, and to aid the body in its natural debridement process. The inflammatory phase can last for up to 3 days, so it may take time for the full degree of tissue damage to present itself. A highly absorbent secondary dressing was used to ensure extra exudate, caused by manuka honey's osmotic action, would be absorbed. A standard three-layer bandage was used to cover the dressings and the patient was hospitalised so neurological examinations could be carried out and analgesia continued.
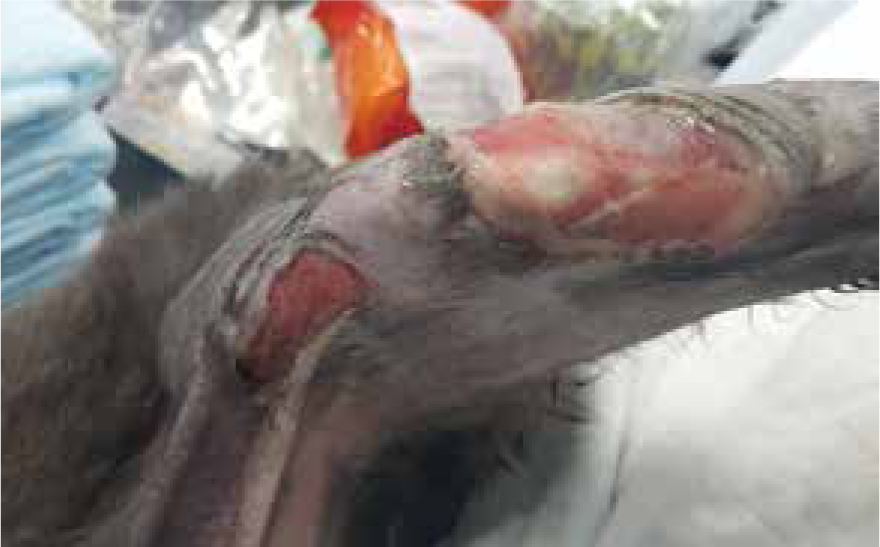
Day 3
Dressing change carried out on day 3 consisted of same process as above, allowing for autolytic debridement with aid of manuka honey.
Day 5
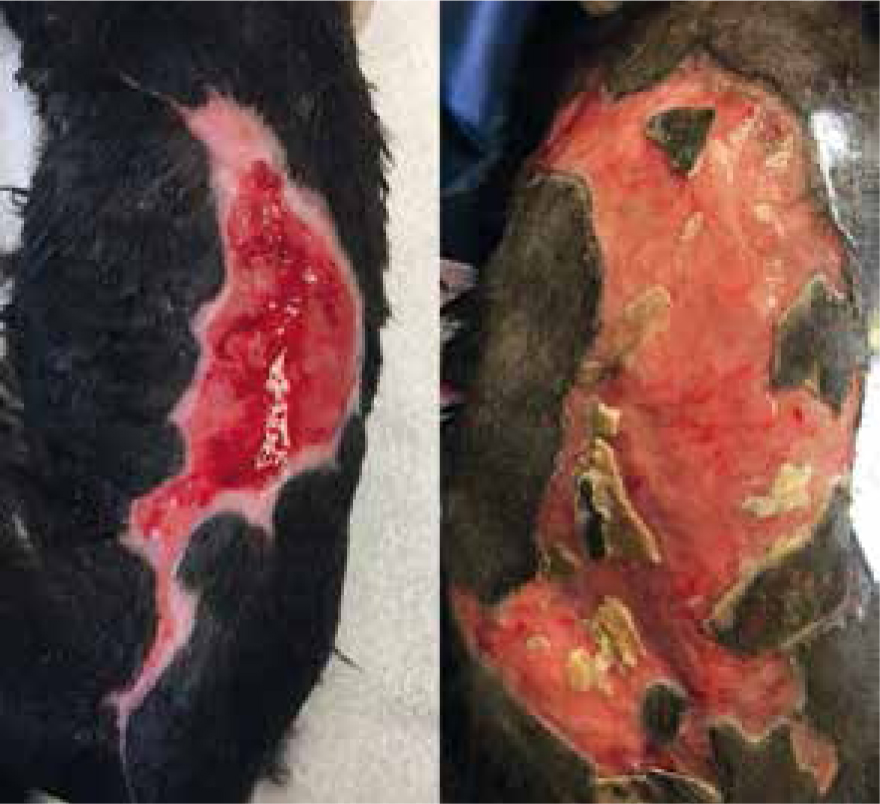
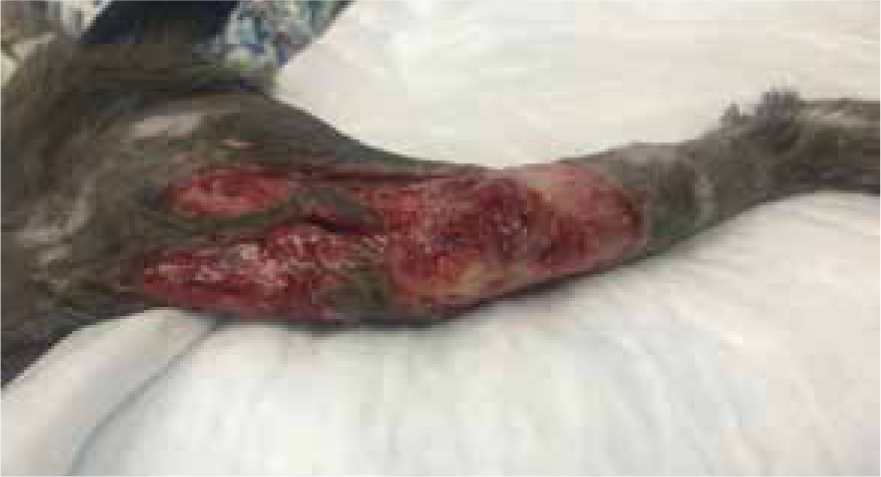
Dressing change exudate levels still quite high, yellow/brown in colour, strike through of absorbent dressing, but not through to other bandage layers. Wound still separated by three margins, but more necrotic tissue present (Figure 11). By allowing the body to perform its natural process, supported by manuka honey, it was possible to more easily identify devitalised tissue for removal via sharp debridement (Figure 12). If this had been opted for this on dressing change one or two, potentially salvageable tissue may have been removed. Care was taken to only remove loose devitalised tissue. Barrier cream (Cavilon, 3M) was applied to the edges of the wound and it was packed with manuka honey Tulle and manuka honey fill tube for extra moisture, ensuring to cover with an appropriate absorbent secondary dressing to accommodate for the expected rise in exudate.
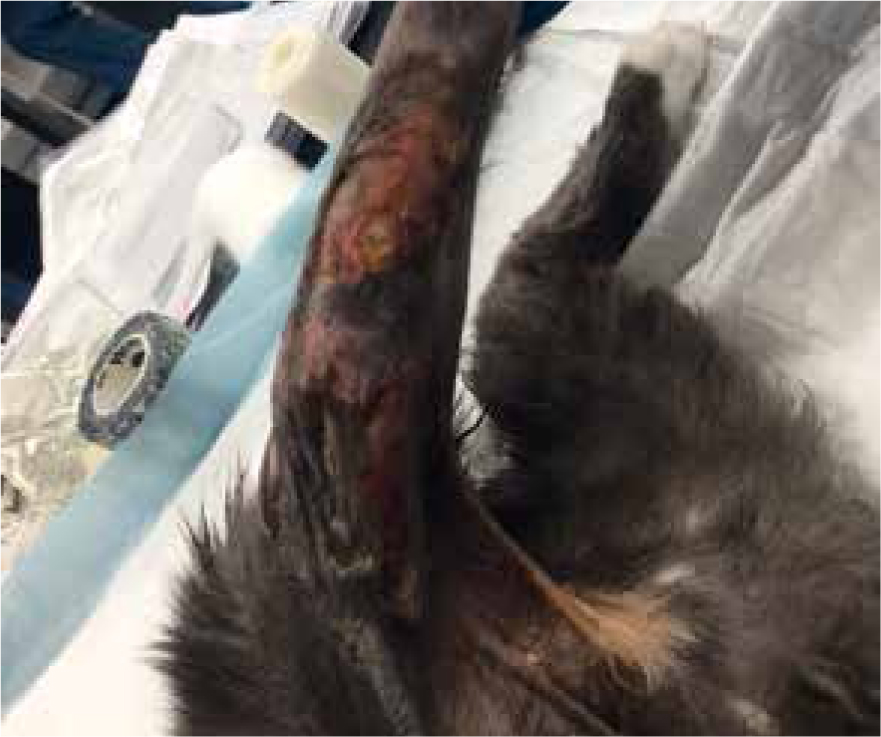
Day 8
Further necrosis has occurred, previously wounds had been separated by three margins, but was now one large wound with small island descending 2 cm from proximal end, measuring 7.8 cm by 11 cm (Figures 13 and 14). Further sharp debridement and mechanical debridement was carried out, using a microfibre pad, to lift embedded devitalised tissue. As with previous dressing change, the wound was lavaged, dried and Cavilon (3M) barrier cream applied to help prevent maceration of healthy tissue, and the wound was dressed using manuka honey Tulle and manuka honey fill tube.
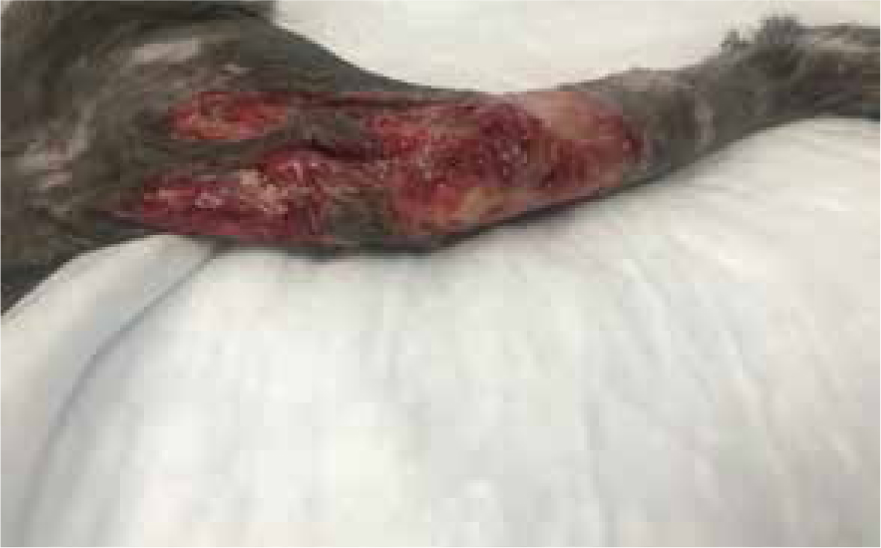
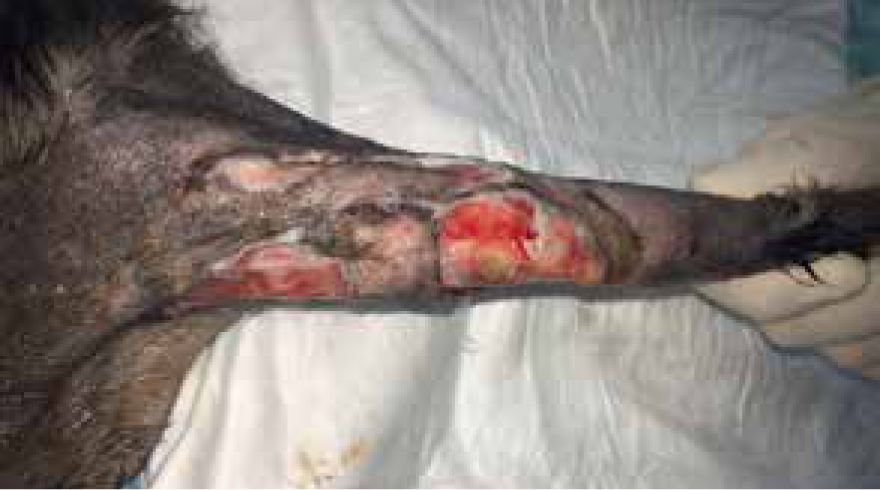
Days 10–33
Bandage changed on day 10 the wound measured 7.5 cm by 11 cm, contraction occurred, the decision was made to change from manuka honey to a hydrophilic dressing to promote moisture and encourage further granulation and contraction (Figure 15), this wound was managed in total for 33 days, as both an inpatient and outpatient (Figure 16).
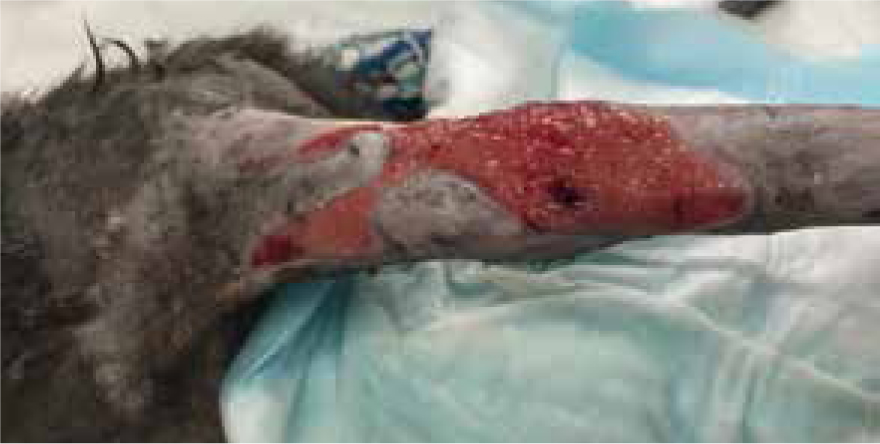
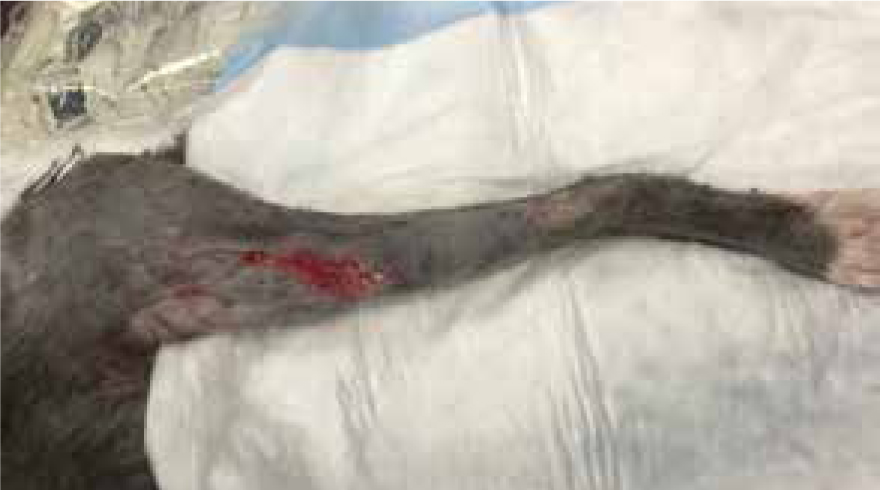
Case study 2: manuka honey on post-operative wound dehiscence
Breed, gender and age:
Canine, Staffordshire Bull Terrier, neutered female, 13 years, BCS 4/5. NB. Initial dressing changes performed using sedation, but consequent dressings were carried out conscious.
Wound location and presentation:
Wound to right lateral abdomen, originally presented with mass to right caudal mammary gland, lump removed with a 10 cm x 15 cm deficit, a single pedicle advancement flap was performed to gain primary closure (Figure 17), an active was drain placed after initial surgery (Figure 18), which was removed 5 days postoperatively due to minimal output. Some swelling was noted at the day 2 postoperative check, and some areas of necrosis around edges of flap at day 4. For the next 12 days the patient was seen as an out-patient, the owner had been massaging the necrotic area with aloe vera gel, which was changed by the veterinary surgeon, on day 16, to silver sulfadiazine cream.
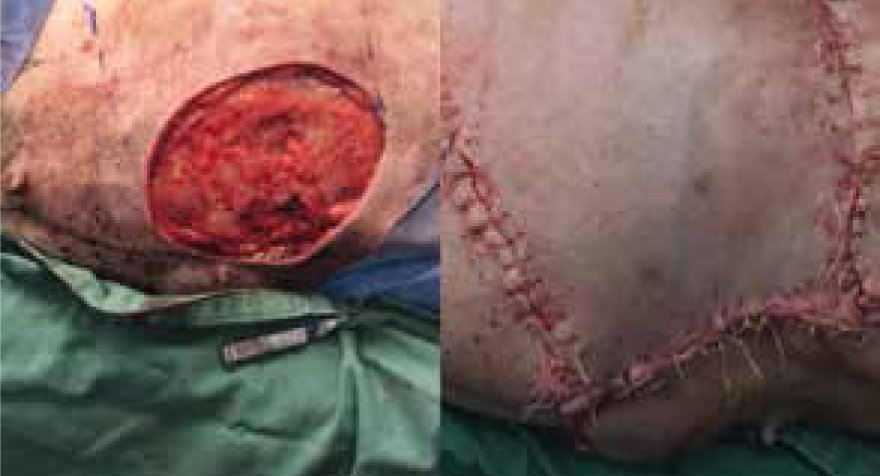
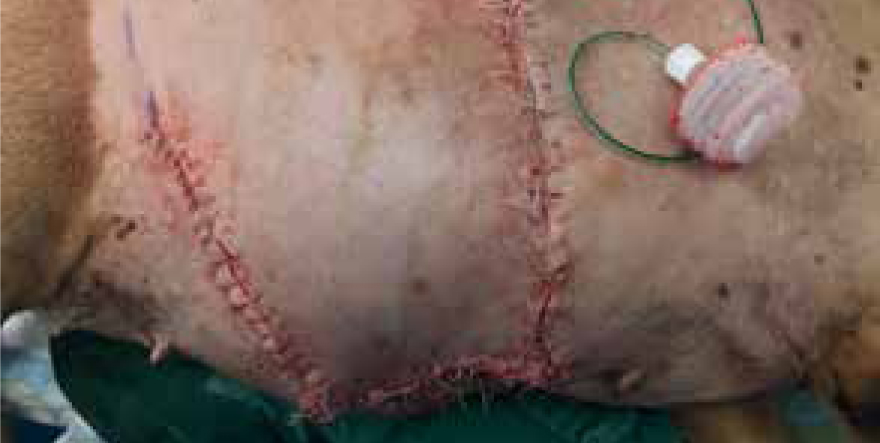
Day 20
Patient was admitted for sedation to further manage the wound dehiscence, the area was clipped, and lavaged; the current deficit measured 14 cm x 5 cm, with necrotic ischaemic tissue covering the granulation bed (Figure 19), non-viable tissue was removed via sharp debridement, exposing a pale, devitalised granulation bed (Figure 20). Cavilon (3M) was applied to the surrounding tissue, tie over sutures placed around the wound bed and manuka honey gel applied for its antimicrobial and osmotic debridement properties to encourage granulation and contraction (Figure 21). This was covered with an absorbent foam dressing, swabs and anchored using 2 cm white open woven bandage. It should be noted that the rest of the flap had already healed, and remaining sutures removed, so issues with further dehiscence at this stage were not a concern.
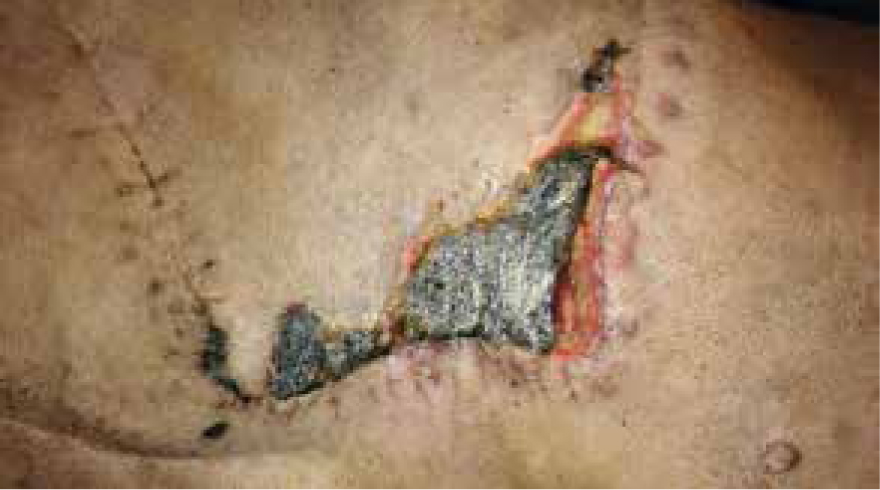
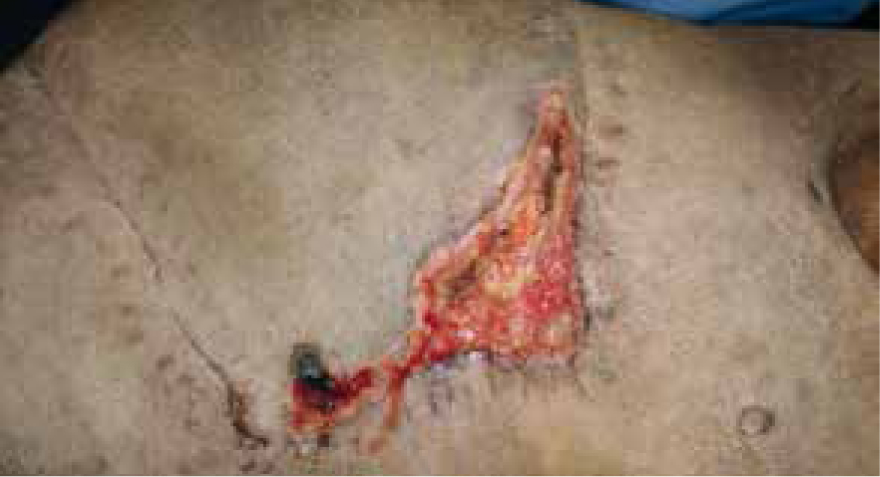
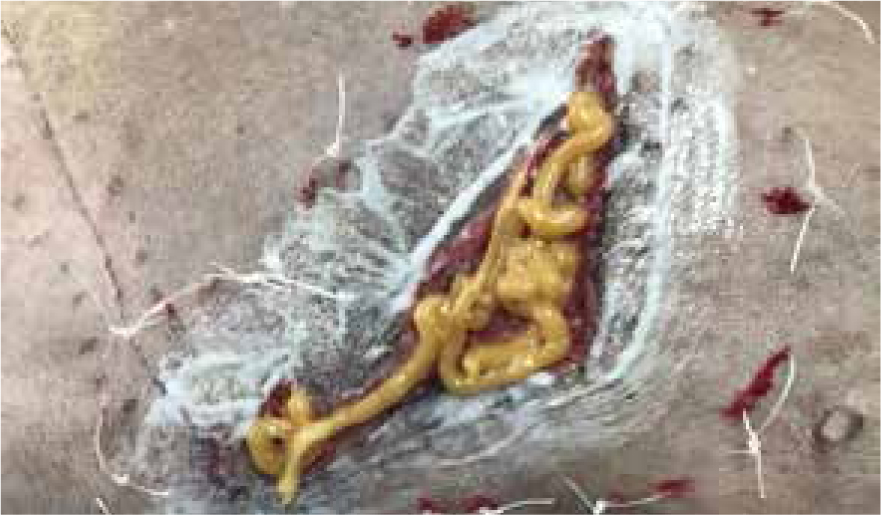
Day 23
Dressing change performed, green and brown exudate present, strike through of foam, very odorous. Measuring 12 cm x 4.5 cm contraction had started and wound bed had started to granulate, but due to the colour of the exudate and the fact that the granulation bed was still pale and macerated manuka honey gel was applied, along with previous dressing technique (Figure 22).
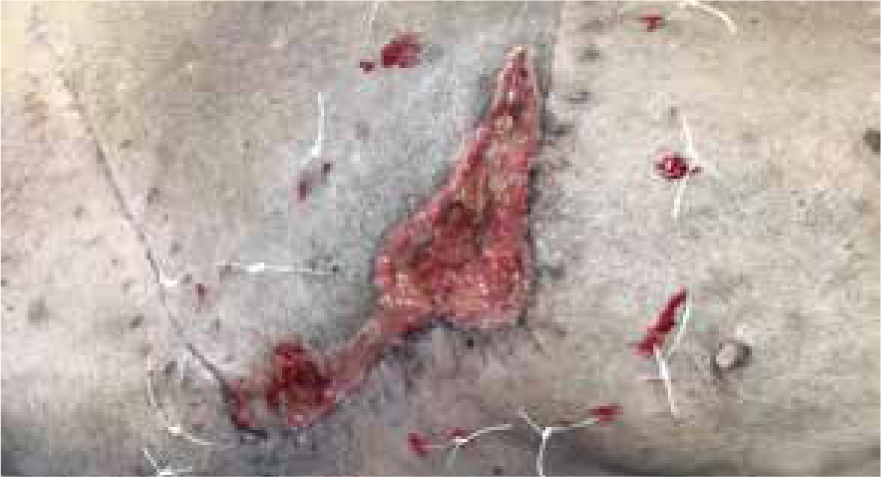
Days 27 and 30
Wound continuing to contract, tie over working well, along with manuka honey for promoting granulation tissue. Same dressing technique applied at both dressing changes.
Days 33–47
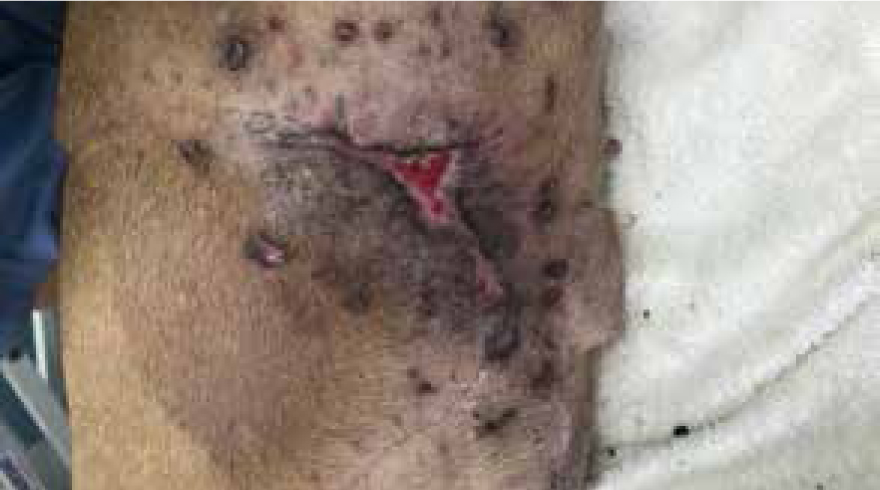
On day 33 the wound was dressed using hydrogel, manuka honey was no longer warranted, wound bed was granulating, with edges continuing to epithelise at each dressing change; day 47 all tie over sutures had been removed and owners had been using barrier cream to massage scar tissue to help with strengthening new skin (Figure 23).
Conclusion
Traumatic, contaminated, infected and necrotic wounds are a regular occurrence in practice. Manuka honey's properties have been demonstrated to aid in the initial inflammatory phase, with its superior antimicrobial activity, when compared with store bought honeys. Manuka provides a way to reduce antibiotic use at a time of increasingly prevalent antibiotic resistant bacteria. The osmotic debridement effect of manuka honey can aid the body in its natural healing process, and provides anti inflammatory effects, which can help to reduce redness, oedema and swelling in chronic troublesome wounds. For use during the inflammatory phase, manuka honey provides a wide range of benefits and should be a staple of every practice's wound management protocols.

