Several causes have been identified for limb amputation in dogs including trauma, bacterial infections, birth defects and appendicular cancer.
Canine appendicular cancer has been reported ‘as an increasingly important disease’ (Segal-Eiras et al, 1982). Osteosarcoma accounts for approximately 85% of canine bone tumours (Boerman et al, 2012), and 8–15% of other tumours classed as subcutaneous soft tissue sarcomas (Dennis et al, 2011). The treatment of choice for these conditions is surgical excision by limb amputation (Boerman et al, 1982; Farese et al, 2009), but limb salvage techniques have also been reported (Lascelles et al, 2005). In this survey 71% of the dogs undergoing limb amputation due to cancerous causes underwent chemotherapy, with 29% of them being treated holistically. Around 60% of the dogs diagnosed with osteosarcoma die in the first year after diagnosis with another 10 to 20% dying in the second year (McCleese et al, 2011).
In an attempt to save their loved pet from the side effects of chemotherapy, owners impelled by compassion, evaluate alternative treatment methods ‘with respect for the body's innate ability to heal itself’ (Kelleher, 2003). Complications of chemotherapy include toxicological effects like neutropenia, thrombocytopenia and anorexia (Kent et al, 2004) and cardiomyopathy (Moore et al, 2007). Holistic treatments include chiropractic, homeopathy, herbal therapy, acupuncture, special diet and massage (Kelleher, 2003).
Several studies have tried to establish a relationship between animal behaviour and pain, particularly in dogs (Conzemius et al, 1997; Firth and Haldane, 1999; Hansen, 2003), leading to the development of different pain scales (e.g. The Melbourne Pain Scale developed by Firth and Haldane in 1999). It has proved difficult to correctly assess degree of pain in animals due to the large number of factors involved, and also due to the anthropomorphisation of scales of assessment (Holton et al, 2001). But in all studies it becomes clear that increased mobility and display of a range of normal behaviours is an indication of lower levels of pain.
It is the aim of this research article to analyse the causes of limb amputation in dogs, identifying predisposition factors. A survival prognosis is also conducted on dogs with diagnosed cancer as the cause of the limb amputation. These aspects can be of fundamental importance in helping the veterinary science professional in costumer counselling and decision making with regards to treatment alternatives.
Materials and methods
Questionnaire design and sample
A questionnaire was designed to allow the collection of data to study the population of dogs having a limb amputation. With the aim to study the epidemiological factors leading to amputation several questions were posed, and after data clearing and cleaning with removal of dubious entries and outliers the following variables were selected for analysis:
Data were collected in 2010, after a questionnaire directed to all the members of the blog Tripawds. com. This blog contains members from all over the world but mainly from the USA, and from these, 152 members, answered the questionnaire.
Statistical methods
Scale type data were tested for normality with the Kolmogorov-Smirnov test and were found to be normally distributed (p>0.05). The prerequisite of homogeneity of variances was also tested and dealt accordingly. The analysis of data was performed between the variables listed above, using the following statistical approaches:
Follow-up data were not available other than for cancer cause, and therefore a survival analysis for cancerous dogs only was performed, where the dependent variable ‘survival time after amputation’ was considered. The independent dummy categorical variables considered were: BCS before amputation, behaviour of the dog in the first week post amputation (animal, vegetal/rock) (see below); type of treatment (chemotherapy, holistic); presence of metastasis after treatment (yes, no); type of cancer (lymphoma, carcinoma, sarcoma, unknown, multiple type); gender (male, female); castration (entire, spayed/neutered. The covariates considered were: age at diagnosis (month), and waiting time between diagnosis and surgery (weeks).
Holistic treatment is the use of alternative and complementary therapies such as chiropractic, homeopathy, herbal therapy, acupuncture, special diet and massage. The dog's behaviour was evaluated as rock, vegetal and animal, corresponding respectively to lack of movement and passive behaviour requiring hand feeding; some movement but always lying down, limited response to stimulus but able to feed himself if food presented; able to stand up and move around, looking for food and water and able to regulate intake according to needs, guard attitude and response to stimulus. For convenience of analysis vegetal was merged with rock behaviour, and therefore ‘behaviour of the dog in the first week post amputation’ was considered to have two levels. With regards to the type of treatment, mixed treatment was disregarded to avoid confounders. After data cleaning and disregarding of causes other than cancer, n = 65 dogs diagnosed with appendicular cancer were used in this study, where dogs still alive were censored (n = 23, 35.4%)
A multivariable Cox regression analysis was implemented and the final adjusted model was obtained via forward stepwise selection of variables, after the Wald test. The −2 Log Likelihood test was used to evaluate the fitness of the adjusted model. Plots of partial survival curves for the different levels of the significantly different variables were also produced. The significance level was set to p<0.05 in all the tests. The analysis was done using the software IBM® SPSS® Statistics 21.
Results
A vast range of dog breeds were included in the amputee analysis; when classed by type, n = 63 (42%) had mixed origin, n = 28 (18 %) were working, n = 32 (21%) sporting, n = 8 (5%) terrier, n = 10 (7%) herding, n = 5 (3%) non-sporting and n = 6 (4%) hound. When classed by size (pure breed only), n = 2 (2%) small, n = 59 (68%) medium and n = 26 (26%) large. With regards to gender, n = 78 (51%) were females and n = 74 (49%) were males. Only n = 7 (5%) of the animals of both gender were not spayed or neutered. With regards to amputee dogs due to cancer, when classed by type, n = 22 (33.8%) had mixed origin, n = 15 (23.1 %) were working, n = 14 (21.5%) sporting, n = 3 (4.4%) terrier, n = 5 (7.7%) herding, n = 3 (4.4%) non-sporting and n = 3 (4.6%) hound. With regards to gender, n = 40 (61.5%) were females and n = 25 (38.5%) were males. The same n = 7 (10.8%) intact dogs of both gender, were counted and therefore all the intact dogs were amputee due to cancer. Using the 3rd edition of the International Classification of Diseases for Oncology, n = 1 (1.5%) of the cancers were classified as lymphoma, n = 5 (7.7%) as carcinoma and n = 44 (67.7%) as sarcoma: n = 38 (58.5%) osteosarcoma, n = 5 (7.7%) fibrosarcoma and n = 1 (1.5%) angiosarcoma. Cancers of unknown n = 9 (13.9%) and multiple type n = 10 (15.4%) were present in the sample.
A Pearson's chi square test for goodness of fit was applied to the frequencies of breeds classified by type and size (only pure breed) in all the amputees and the cancerous only and no significant differences (p>0.05) were found. Therefore no predisposition of breeds to cancer was made evident.
A Pearson's chi square test for independence using gender (male, female) and causes of amputation (cancer, trauma) found a positive association (p< 0.05) between female dogs and cancer, suggesting that females are more prone to cancer than males; birth defects and infections were disregarded from this analysis.
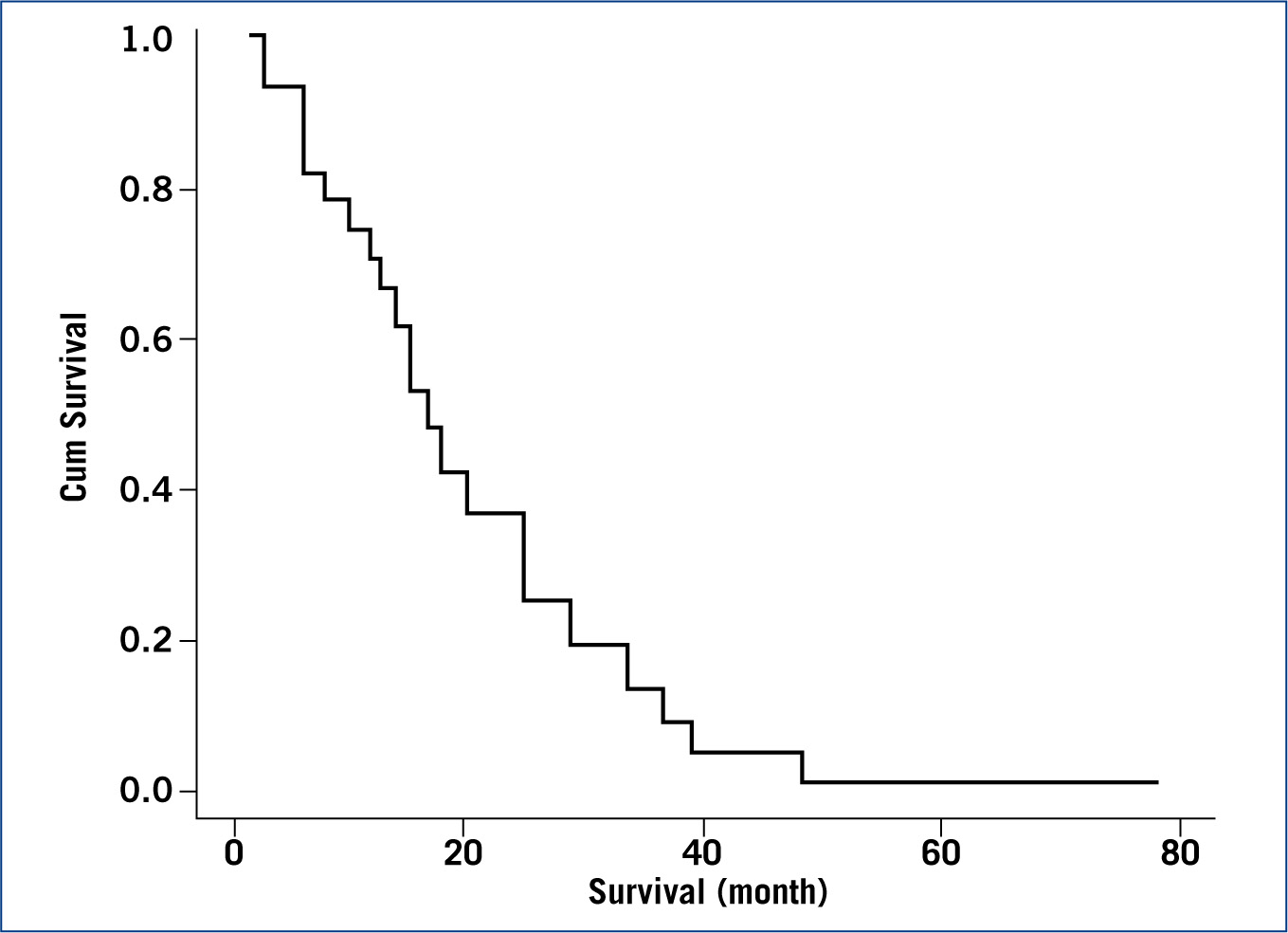
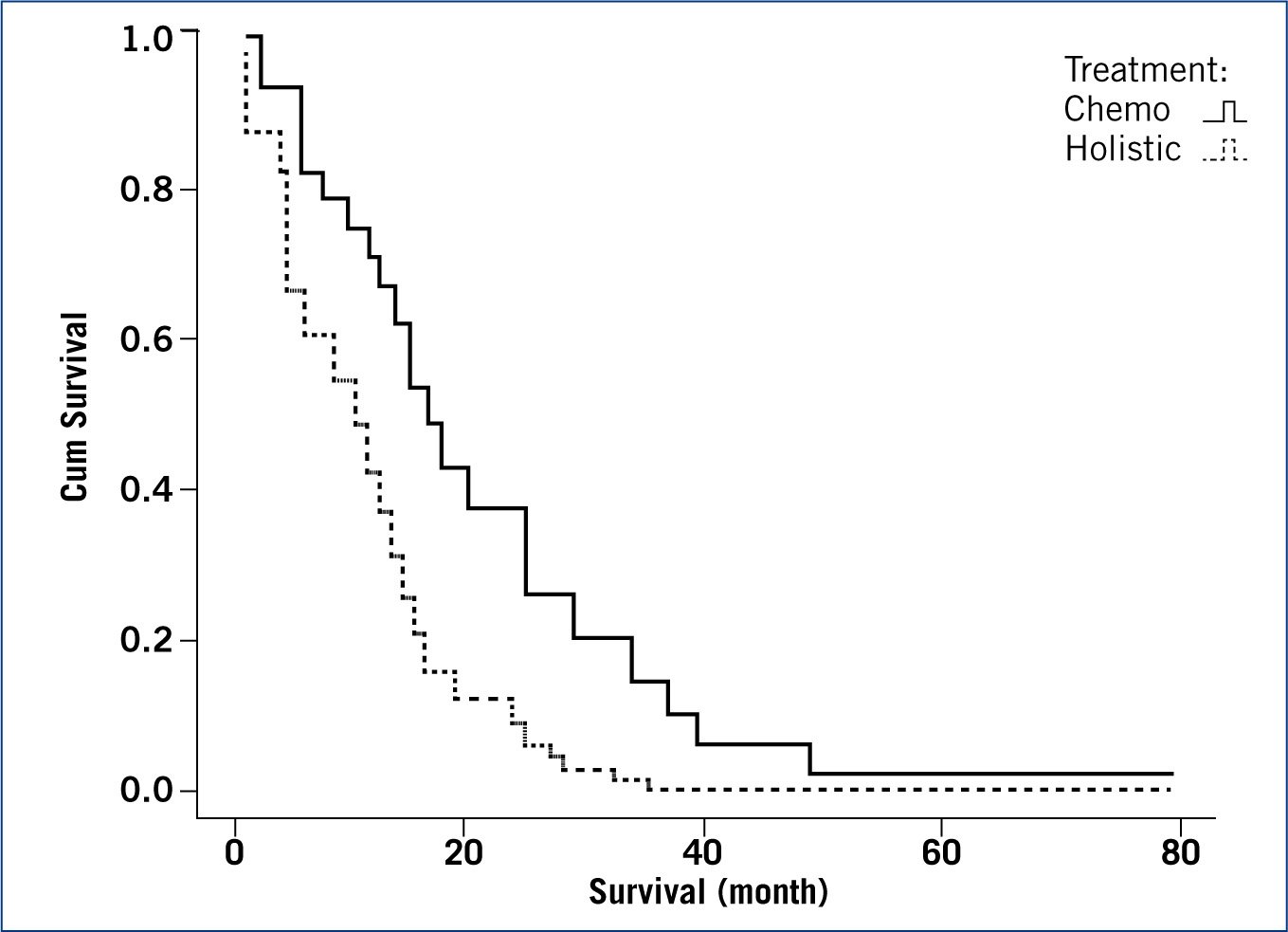
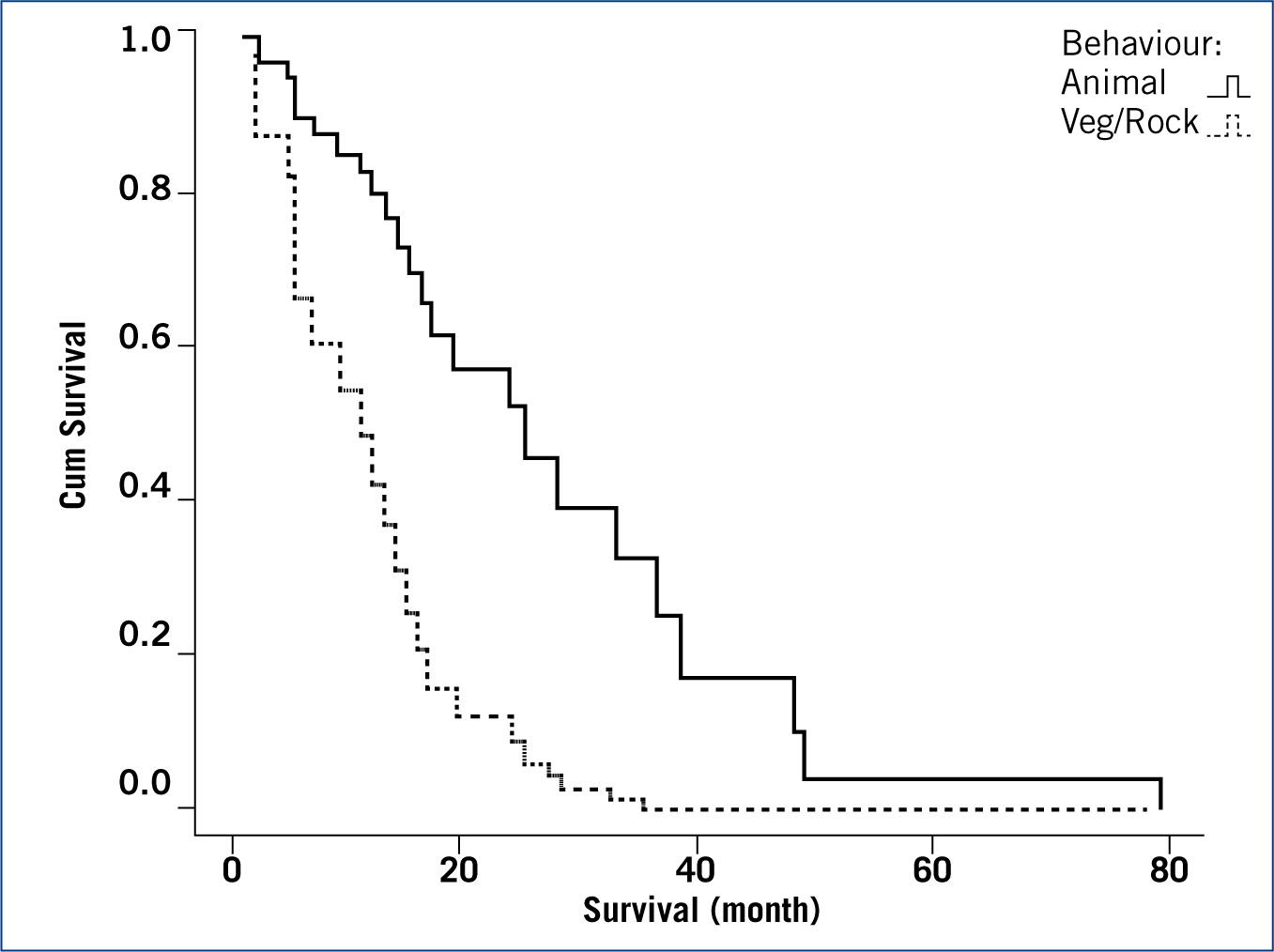
A Cox regression model was successfully fitted (p< 0.01) with a −2 log likelihood of 115. The significant variables found were ‘treatment’ (chemotherapy, holistic) (p<0.05) and ‘dog behaviour in the first week after amputation’ (animal, vegetal/rock) (p<0.05). Dogs treated by chemotherapy (mean = 23.5; median = 16 month) were seen to have a longer survival time than those treated holistically (mean = 11.1; median = 4.5 month); and dogs showing an animal behaviour in their first week after amputation were also seen to survive longer (mean = 23.2; median = 14 month) than those showing vegetal/rock behaviour (mean = 9.8; median = 8 month). The full description of the model coefficients is summarised in Table 1. Figure 1 plots the survival function at means of variables and Figures 2 and 3 plot the partial survival functions for the different levels of the variables found significant.
A Fisher's exact test of independence, using behaviour (animal, vegetal/rock) and treatment (holistic, chemotherapy) as nominal variables, have also shown to be significant (p<0.05), with dogs displaying animal behaviour being mainly treated by chemotherapy and the others, holistically.
With respect to cause of amputation, significant differences were found for age at diagnosis (p<0.05), when considering trauma (mean 3.98 years, 95% confidence interval (3.21; 4.75)) and cancer (7.66, (7.41; 8.01)), and therefore traumatic causes of amputation are diagnosed earlier in the life of dogs and cancerous causes later.
With regards to cancerous dogs only, those showing an animal behaviour (6.17, (5.78; 6.56)) in the first week after amputation are diagnosed younger (p<0.01) than dogs showing a vegetal/rock behaviour (7.70, (7.34; 8.06)). Significant differences were also found for spayed/neutered and entire dogs (p<0.05). Intact animals tended to have an earlier diagnosis of appendicular cancers (2.79, (0.86; 4.72)) than spayed/neutered animals (7.69, (7.36; 8.02)). These results need to be interpreted with care as the samples are highly unbalanced (58 spayed/neutered against seven only intact), and the intact dogs sample is very small; using the non-parametric Mann-Whitney U test the analysis is not significant (p>0.05).
Discussion
With regards to gender of amputee dogs, no significant differences (p>0.05) were found for age at diagnosis. An association between females and cancer was found to be significant (p< 0.05) when considering the proportion of amputees due to cancer or trauma, with the former having a higher probability in relation to males there is definitively a relation between females and cancer. Misdorp and Hart (1979) reported 30 years ago that sarcomas in females are larger in size than those observed in dogs, however it should be kept in mind that the present study refers to sarcomas in the limbs leading to limb amputation. Anfinsen et al (2011) found no gender predisposition in bone tumours in agreement with Rosenberger et al (2007) and Ru et al (1998), but these authors refer to several others where predisposition in females to osteosarcoma was reported in Saint Bernards (Brodey and Rise, 1969), and axial osteosarcoma predisposition was reported indiscriminately in females (Heyman et al, 1992); reports indicating a male predisposition to osteosarcomas, arising mainly in limbs, were also found (Brodey and Abt, 1976; Misdorp, 1980). The most conclusive study, however, can be considered as that made by Bonnett et al (2005) and the companion paper Egenvall et al (2005) using a sample of 350 000 dogs where a mortality rate ratio (RR) female/male of 1.1 was reported for tumour and 0.8 for trauma, therefore, favouring predisposition of females to tumours and males to trauma; these results are in complete agreement with those of the present study (3.1 and 0.32 respectively). Note, however, that the other authors' results were related to all causes of death including mammary cancer in females and the present study refers to cases leading to limb amputation. Munro and Thrusfield (2001) found that non-accidental inflicted traumatic injuries are more common in males, justified by the higher degree of aggressiveness in males, resulting in the predisposition of males to trauma leading to limb amputation.
In this study age at diagnosis for amputation has a mean value of 6.9 (6.63; 7.17) years, and 7.16 (6.76; 7.56) years for cancerous dogs, which agrees with Anfinsen et al (2011) and sits between Farese et al (2009) (6.58 years) and Bhandal and Boston (2011) (8.84 years), all referring to dogs with diagnosed osteosarcoma, the first with limb predilection and the former two in the limbs only. It should be noted, however, that the present study includes all types of cancer leading to amputation, with osteosarcoma being the most frequent (58.5%) form of cancer.
The effect of castration on age at diagnosis was found not to be significant (p>0.05) when considering all the amputation causes, but was found to be significant in cancerous dogs: neutered/spayed dogs were diagnosed at a later stage in their lives (7.69, (7.36; 8.02) years) than intact dogs (2.70, (0.86; 4.72) years). The decrease of incidence of mammary gland related neoplasia, in older dogs is well documented (Root-Kustritz, 2007). Several studies have also shown an increased risk of osteosarcoma in neutered dogs, especially when this procedure is performed at an early age (Ru et al, 1998; Cooley et al, 2002; Root-Kustritz, 2007). This is not in agreement with the author's findings where the opposite was found. Although, as indicated previously, these findings are reported with reservations.
With regards to the age at diagnosis, significant differences were found between cancerous (7.66, (7.41; 8.01) years) and traumatic causes (3.98, (3.21; 4.75) years), and therefore cancerous causes of limb amputation happen later in the dogs' life than traumatic causes. This result makes sense, as it can be expected for cancer to show up more frequently in advanced age, and accidents to happen in younger inexperienced animals. Munro and Thrusfield (2001) indicated that younger animals have a higher predisposition for non-accidental inflicted traumatic injuries than older animals; and the conclusive study made by Bonnett et al (2005) and Egenvall et al (2005) also reported that predisposition to traumatic death decreases with age while predisposition to cancer increases with age.
Survival analysis of dogs diagnosed with cancer, revealed that treatment (p<0.05) and behaviour (p<0.05) were significant factors. Dogs treated with chemotherapy (median 16.0 month, 95%CI (10.5; 21.5)) have been shown to survive longer than dogs treated holistically (4.5 (0.7; 8.3)). Dogs showing animal behaviour in the first week post amputation (14.0 (7.5; 20.5)) have been shown to survive longer than dogs showing vegetal/rock behaviour in the first week (8.0 (4.1; 11.9)).
Treatment with chemotherapy (carboplatin, cisplatin, and doxorubicin) has been shown previously to double survivability in dogs after surgical removal of a limb due to osteosarcoma (Coomer et al, 1996) in single agent protocols (Berg, 1996; Phillips et al, 2009) and in protocols using two agents (Chun et al, 2000; Kent et al, 2004). Carboplatin was the drug of choice in the sample used in this study, in single drug protocol or in combination with doxorubicin, some dogs were also treated with gemcitabine and lomustine. The results of this study are, therefore, not surprising.
With regards to the behaviour in the first week after amputation surgery, the results are also as expected. A dog developing an animal behaviour, showing mobility and general activity as opposed to daytime sleeping, indicates the presence a lower degree of pain and discomfort (Wiseman et al, 2001) which, in this study is found to be associated with higher survival rates. The significant (p<0.05) association between treatment by chemotherapy and animal behaviour is a reiteration of the survival analysis.
Dogs showing an animal behaviour (mean 6.17 years 95%CI (5.78; 6.56)) have also been shown to be diagnosed significantly (p<0.01) younger than dogs showing a vegetal/rock behaviour (7.70 (7.34; 8.06)), and despite the fact that age at diagnosis was found not to be significant in the survival analysis, younger amputees can be expected to survive longer; it is likely these cases were diagnosed at an early stage of the cancer.
Several authors (Bonnett et al, 2005; Egenvall et al, 2005) have identified a relationship between large dog breeds and development of osteosarcoma. The present study did not identify any breed effect, however it should be noted that this study refers to all types of cancer leading to limb amputation.
Conclusion
In conclusion this study found an association between amputee bitches and cancer and amputee dogs and trauma. Castrated/spayed dogs were diagnosed for amputation later in their lives, when compared with intact animals; cancerous causes were also diagnosed later in their lives, when compared to traumatic causes leading to amputation. The survival analysis of amputee cancerous dogs showed an advantage of treatment by chemotherapy over holistic approaches and of animal over vegetal/rock behaviour in the first week after amputation. An association was also found between animal behaviour and treatment by chemotherapy which reiterates the survival analysis results. This information can be used by practitioners in decision making with respect to which treatment strategies to adopt; it is therefore also important information to be used in owner counselling.

