Hypoadrenocorticism (Addison's disease) is often called the great pretender because it usually presents with nondescript signs and symptoms. As a result it may be misdiagnosed which can cause a delay in treatment and a life-threatening condition. Although it is uncommon in dogs and even rarer in cats it is important to understand this disease, signs and how to treat to allow patients the best chance of survival and long-term success (Norkus, 2012).
The adrenal glands
The body contains two adrenal glands which lie close to the kidneys. The adrenal glands are part of the endocrine system and help to produce several hormones. Glucocorticoids are released by the adrenal glands and are regulated by adrenocorticotropic hormone (ACTH) from the pituitary gland (Aspinall and O'Reilly, 2004). The term corticosteroids is applied to hormones of the glucocorticoid class (those that affect glucose) that are released from the adrenal glands (Aspinall and O'Reilly, 2004).
The most important corticosteroids produced are cortisol and corticosterone. They help increase blood glucose levels by reducing glucose uptake in the cells, aid in gluconeogenesis and help to convert fatty acids back to glucose. When there is an excessive amount of corticosteroids the inflammatory and immune process can be reduced, which can lead to poor healing (Aspinall and O'Reilly, 2004; McGavin and Zachary, 2007).
The adrenal glands also produce aldosterone, a mineralocorticoid (affects ‘minerals’ also known as electrolytes) which regulates acid/base function of the kidney (Aspinall and O'Reilly, 2004). It also helps to control the excretion of sodium, chloride and potassium by the kidneys (Aspinall and O'Reilly, 2004; McGavin and Zachary, 2007).
Lastly the adrenal glands are responsible for producing both male and female sex hormones and while they produce seemingly insignificant quantities, it may explain why some animals still display some level of sexual behaviour even after being neutered (Aspinall and O'Reilly, 2004).
Adrenal insufficiency
The production of mineralocorticoids, corticosteroids and/or sex hormones can decrease for a variety of reasons. Causes include bacteria or parasitic agents (histoplasma, cryptococcus) causing inflammation in the adrenal glands, haemorrhage (Waterhouse-Friderichsen syndrome due to massive sepsis), neoplasia, iatrogenic causes or may be idiopathic (Battaglia, 2007; McGavin and Zachary, 2007; Norkus, 2012). Most dogs diagnosed with hypoadrenocorticism are young to middle-aged females (mean 4–5 years), with some reporting that 70% of dogs diagnosed with hypoadrenocorticism fall into this description (Scott-Moncrieff, 2011). Certain breeds seem to have a higher risk: Standard Poodles, West Highland Terriers, Bearded Collies, Great Danes, Portuguese Water dogs, Labrador retrievers, Rottweilers, and Wheaton Terriers (Kahn, 2010; Norkus, 2012).
Roughly 90% of the adrenal function is compromised once clinical signs of Addison's disease finally become evident (Norkus, 2012). As the mineralocorticoids are decreased the sodium, chloride and potassium levels within the body become unregulated. The kidneys no longer secrete appropriate amounts of potassium which leads to hyperkalaemia (McGavin and Zachary, 2007). In addition, the renal tubules do not reabsorb sodium and chloride resulting in both being excreted rapidly in the urine. This leads to a decrease of these in the blood (McGavin and Zachary, 2007). Aldosterone has a direct effect on the extracellular fluid because of its direct relationship with how much sodium is reabsorbed or excreted (Hall, 2011). In patients with hypoadrenocorticism extracellular fluid volume will decrease as sodium decreases, thus causing the patient to become dehydrated (Wingfield and Raffe, 2002). Any small change in sodium, even by a few milliequivalents on blood work, will stimulate thirst and water intake (Hall, 2011).
The hyperkalaemia will often cause electrocardiogram (ECG) changes resulting in high-peaked T waves (Figure 1) and bradycardia (Murtaugh, 2002; McGavin and Zachary, 2007). It can also cause abdominal pain, weakness (due to the cardiac effects), diarrhoea and in severe cases flaccid paralysis of the limbs, respiratory paralysis, circulatory and cardiac failure (Murtaugh, 2002; McGavin and Zachary, 2007).
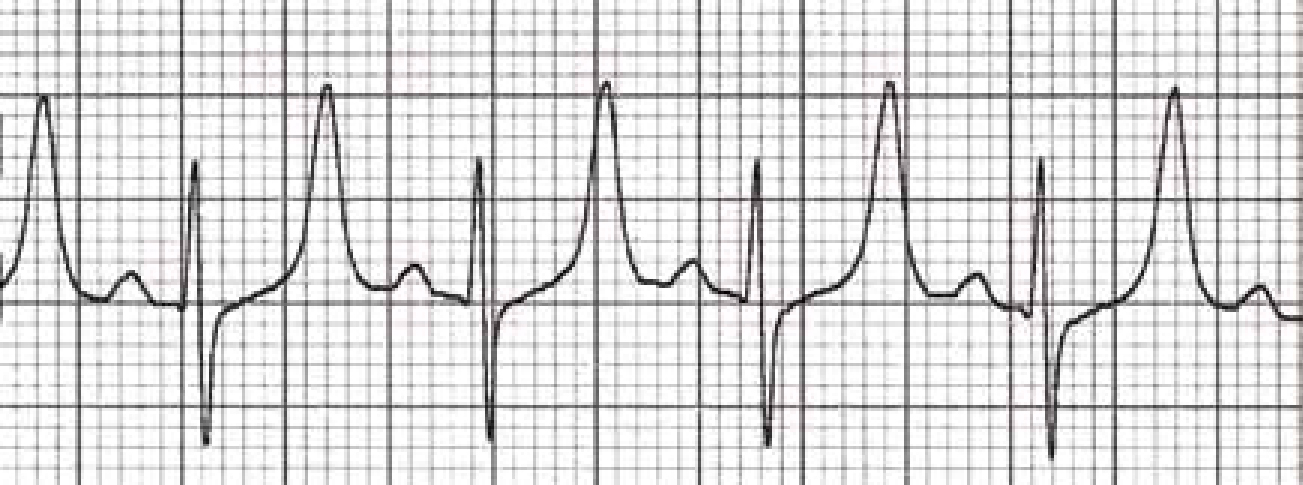
As the sodium decreases the pet will become depressed, lethargic and cerebral oedema may occur (Murtaugh, 2002). Seizures and coma may occur although these signs are often a reflection of how fast the sodium declined rather than the actual concentration (Murtaugh, 2002). If the sodium decreases slowly enough the pet may have few clinical signs (Murtaugh, 2002).
As the corticosteroid levels decrease gluconeogensis may not occur which can result in a mild to moderate hypoglycaemia (McGavin and Zachary, 2007). This can lead to more lethargy of the pet.
Signs/symptoms
Hypoadrenocorticism may occur acutely or chronically. If the animal has time to adapt to the shifting electrolytes and decrease in hormones then they typically present with more non-descript signs: weight loss, hypothermia, shivering, weakness, anorexia, lethargy (Figure 2), vomiting, polyuria and polydipsia (Wingfield and Raffe, 2002; Battaglia, 2007). Some patients will also have weight loss, diarrhoea and melena (Battaglia, 2007). Cats typically experience lethargy, anorexia, vomiting, weight loss, polyuria and polydipsia (Battaglia, 2007). When the body does not have a chance to get adapt to the decreases in hormones the animal typically presents with severe dehydration, hypovolemic shock, collapse and bradycardia (Wingfield and Raffe, 2002).
On presentation pets are usually depressed and at least mildly dehydrated (Battaglia, 2007). Their owners may report waxing and waning signs of anorexia, lethargy and occasional bouts of vomiting and urinary accidents in the house (or frequent urination). Because of this hypoadrenocorticism is frequently overlooked.
Pets that experience an acute onset of hypoadrenocorticism will often present with signs of hypovolemic shock: pale/tacky oral membranes, decreased capillary refill time, weak pulses, bradycardia, shaking, collapse, seizures or coma. These patients require emergency treatment in order for survival to occur.
Diagnosis
Because hypoadrenocorticism is the ‘great pretender’ it can often be hard to diagnose. The key is to be looking for it so that appropriate testing can be done to diagnose it in a timely manner. A complete blood count (CBC), blood chemistry panel, electrolyte panel, blood gas and ACTH stimulation test are all necessary if Addison's is suspected (Norkus, 2012).
Typically a mild anaemia may be present, but may be masked by dehydration (Wingfield and Raffe, 2002). The CBC may also reveal a lymphocytosis and/or an eosinophilia, but is more typically normal (Wingfield and Raffe, 2002; Norkus, 2012). On blood chemistry a hyponatraemia, hyperkalaemia and a sodium/potassium ratio less than 27:1 is usually present (Wingfield and Raffe, 2002). Azotaemia, hypocalcaemia and hypoglycaemia are present in about 30% of pets (Norkus, 2012). Pets may also experience metabolic acidosis and have a urine specific gravity <1.030 (Wingfield and Raffe, 2002).
Definitive diagnosis requires the ACTH stimulation test (Wingfield and Raffe, 2002). ACTH is a hormone produced in the pituitary gland that stimulates the adrenal glands to release cortisol and aldosterone (Graham, 2009). During the test, a small amount of synthetic ACTH (cosyntropin, cortrosyn) (Figure 3) is injected either via intramuscular (IM) or intravenous (IV) routes, and the amount of cortisol the adrenal glands produce in response is measured (Graham, 2009). In pets with hypoadrenocorticism, both the pre- and post-ACTH cortisol concentrations are usually less than 1 µg/dl, with some references stating that under 2.5 µg/dl is diagnostic (Kahn, 2010; Scott-Moncrieff, 2011). Usually the resting cortisol (the pre sample) is <2.5 µg/dl which indicates a need for the stimulation test (Kahn, 2010).
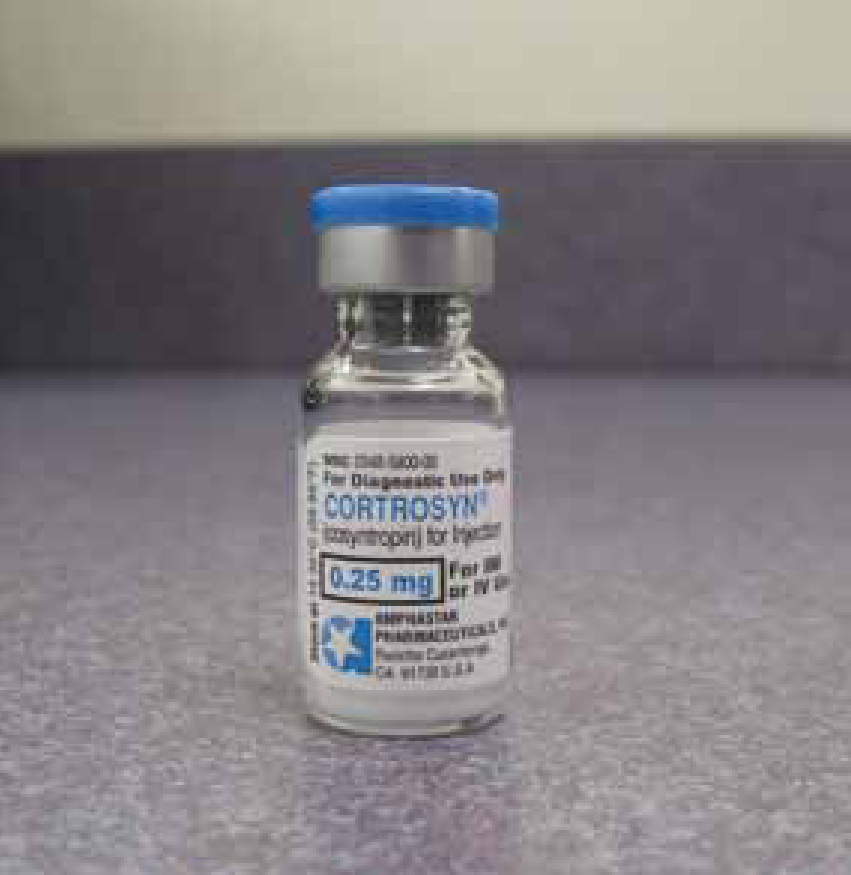
Treatment
An adrenal crisis is an acute medical emergency and requires the staff to work quickly in order for the patient to have the best chance of survival. Dealing with the Addisonian crisis requires treating the patient's shock. Focus should be on treatment of the hypotension and hypovolemia (Norkus, 2012).
A short, large gauge IV catheter should be placed and fluid therapy started. Since the patient is usually hypotensive and vasconstricted blood should be taken via the IV catheter once it is placed as subsequent venipuncture may prove challenging. This will allow for blood work to be run at the same time that fluid therapy is initiated. If hypoadrenocorticism is suspected then 0.9% NaCl should be considered because it offers a higher amount of sodium and lower amount of potassium compared with other isotonic crystalloids (LRS, Normosol-R) (Battaglia, 2007; Norkus, 2012). If the patient is profoundly hyponatraemic caution should be taken as a rapid rise in sodium can result in detrimental neurologic effects due to osmotic injury of the neurons (Hall, 2011). Any balanced isotonic crystalloid is an acceptable fluid choice in most Addison's disease patients (Norkus, 2012).
The patient may need to be started on dextrose supplementation if they are hypoglycaemic (Norkus, 2012). If a patient is severely hypoglycaemic an initial bolus of dextrose should be administered (1:1 solution mixed with 0.9% NaCl given slowly IV) (Kahn, 2010). The patient can then be placed on a 2.5–5% dextrose constant rate infusion (CRI) until they are able to regulate their glucose appropriately (Norkus, 2012).
Patients with severe hyperkalaemia (severe brady-cardia, ECG abnormalities), should be given regular insulin to help drive the potassium back into the cells and then immediately given IV dextrose to help combat the decrease in blood glucose caused by the insulin (Battaglia, 2007). Patients should then be placed on a 2.5–5% dextrose CRI until the insulin's effects have ceased and the patient is able to regulate their glucose. Calcium gluconate can also be given. While it does not decrease serum potassium levels it does protect the heart against the effects of hyperkalaemia (Battaglia, 2007). Typically these treatments are not needed as serum potassium is usually lowered fast enough through the dilution effects from the IV fluids.
Bicarbonate therapy is usually not necessary as the metabolic acidosis is usually mild. However, if the bicarbonate level remains less than 12 mEq/l even after fluid resuscitation, then bicarbonate administration should be considered. Administration of bicarbonate can result in coronary acidosis, a paradoxical central nervous system acidosis and therefore sudden death (Norkus, 2012). If the patient is not responding to initial fluid resuscitation efforts and still in a critical acidotic state then bicarbonate therapy can be initiated. Bicarbonate must be administered slowly (over 2–6 hours) to avoid overwhelming the blood–brain barrier with rising levels of PCO2 (Norkus, 2012).
If the patient is stable or after they are stabilised the focus should shift to providing glucocorticoid replacement. Starting glucocorticoid therapy will also help with electrolyte imbalances. All glucocorticoids react with the ACTH stimulation test except dexamethasone (Wingfield and Raffe, 2002; Battaglia, 2007). Ideally blood for the ACTH test should be performed before glucocorticoids are given. If the patient is not stable before the ACTH test can be completed then dexamethasone sodium phosphate should be given IV (Norkus, 2012). Dexamethasone is long acting, about 30 times more potent than hydrocortisone, but offers no mineralocorticoid activity (Plumb, 2011).
If the patient is stable enough for the ACTH test to be completed then prednisolone sodium succinate is the glucocorticoid of choice (Kahn, 2010; Norkus, 2012). Prednisolone is a glucocorticoid that has some mineralocorticoid activity (Plumb, 2011). Some suggest hydrocortisone sodium succinate be administered instead of prednisolone because of its combination of mineralocorticoid and glucocorticoid activities (Church, 2009; Panciera, 2009). Hydrocortisone has only 25% of the glucocorticoid potency of prednisolone, but offers more mineralocorticoid effects than prednisolone (Church, 2009).
Other treatments should be tailored to the pet's condition. Antibiotics should be considered in patients with severe melena or haemorrhagic diarrhoea. Antiemetics (metoclopramide, maropitant) can be administered to help combat nausea and vomiting. Colloids may need to be administered in patients with hypoalbuminemia and hypotension (Mazzaferro, 2008).
Nursing care
Monitoring the hospitalised patient with hypoadrenocorticism is very important. Patients should be monitored for signs that they are not responding to the glucocorticoid therapy. This may include weakness, lethargy, anorexia, vomiting, ataxia, hypotension and/or tachy- or bradycardia. Physical examination parameters (heart rate, pulse, respiratory rate effort, mucous membranes, capillary refill time and blood pressure should be checked every 2–6 hours depending on how critical the patient is. If the patient is very critical it may be placed on continuous oscillimetric blood pressure monitoring or pressures may be checked constantly until the patient is normotensive. If the patient was experiencing ECG abnormalities then they should be placed on continuous ECG monitoring or have periodic ECG checks minimally every hour or two.
Patients with hypoadrenocorticism should be weighed twice a day to monitor rehydration (Battaglia, 2007). Urine output should also be monitored to ensure the kidneys are functioning properly as well as for fluid therapy. This can be done by placing a urinary catheter or free catching urine for quantification (Figure 4). Urine output should be close to or equal to that of the amount of intravenous fluids going into the patient. Due to the severity of dehydration urine output may be lower than volume input until the patient is adequately hydrated (Battaglia, 2007).
Blood pressure monitoring is extremely important in hypoadrenocorticism patients and should be based on the patient's condition, but should be monitored minimally every 4–6 hours. If the mean arterial pressure (MAP) falls below 60 mmHg, the kidneys and other organs are not appropriately perfused putting them at risk for organ failure. Normalisation of blood pressure, defined by a MAP of 80–120 mmHg or systolic between 110–160 mmHg, is the goal (Bryant, 2009).
Electrolytes, renal function and glucose should all be monitored regularly to assess the response to the glucocorticoid therapy (Kahn, 2010). Initially electrolytes should be rechecked every 4–6 hours (Battaglia, 2007). Once stable electrolytes should be rechecked every 12–24 hours (Battaglia, 2007). Once the patient is on long-term corticosteroid therapy rechecks will occur every 2–4 weeks until the patient is stable (Battaglia, 2007). Because of the repeat blood draws a central line should be considered in some patients. Placement of a central line would allow for frequent blood draws with less trauma to the veins of the patient as well as monitoring central venous pressures in patients who prove challenging to stabilise.
It is important that patients must be given appropriate nutrition. Nurses need to tempt patients to eat and alert the veterinarian if the patient is nauseous or experiencing vomiting or anorexia. It is important to record the amount and type of food the patient has eaten. Feeding tubes, syringe feedings or parental nutrition should be considered in patients that are unwilling to eat.
Long-term care
Many owners of Addisonian pets will start to pick up on the subtle changes their pets will show immediately before a crisis. It is imperative not to dismiss the owner's concerns. ‘Missing a meal’, ‘appears more tired’ or ‘vomited once’ may all be signs that the pet is about to have a crisis or is unregulated on its medication.
With diligent monitoring by the owner and appropriate medication hypoadrenocorticism has a good prognosis (Wingfield and Raffe, 2002). Therapy is required for life and dosage may need to be altered throughout the pet's lifetime. Some clients may not be able to afford the cost of the medication and may elect euthanasia. It is important that owners understand the pet's need for regular veterinary visits, recheck of blood work and medication costs.
Patients will most likely need to be given both mineralocorticoid and glucocorticoid supplements for the rest of their lives. Oral prednisone or prednisolone is the glucocorticoid treatment of choice in both dogs and cats (Figure 5) (Kahn, 2010; Norkus, 2012). In times of stress the prednisone may need to be increased (Norkus, 2012). It is important that following stabilisation the dose is tapered to the lowest effective dose as high doses may result in hyperadrenocorticism (Scott-Moncrieff, 2011). Doses should be decreased 50% per week (over 3–4 weeks) until they can ideally be weaned off, or the pet starts to show signs of glucocorticoid deficiency (Norkus, 2012). The decision to use glucocorticoids is based mainly on gastrointestinal signs (Battaglia, 2007).
There are two options for mineralocorticoid replacement: fludrocortisone acetate (Florinef, Bristol-Myers Squibb Company) or desoxycorticosterone pivalate (DOCP) (Norkus, 2012). The most economical option is DOCP (Percorten-V, Novartis) which requires the pet to have an injection every 21–30 days (Plumb, 2011). It does not have any glucocorticoid activity (Plumb, 2011). Almost all dogs on DOCP require prednisone at least every other day whereas only 50% of dogs on fludrocortisone require supplemental prednisone (Battaglia, 2007; Scott-Moncrieff, 2011).
Fludrocortisone is dosed orally and is given twice a day. It contains both glucocorticoid and mineralocorticoid properties (Norkus, 2012). It is more expensive and there is a commitment to daily medication on the owner's part (Norkus, 2012). The dose of fludrocortisone is more precise and can be tapered more quickly to the pet's needs (Hess, 2012). This may be helpful for a pet that does not regulate well on DOCP.
Both DOCP and fludrocortisone should be tapered to effect. Typically fludrocortisone needs to be increased over time, while DOCP is usually decreased over time (Scott-Moncrieff, 2011). Blood work needs to be rechecked every 3–6 months once the proper dose has been determined (Battaglia, 2007). Owners should be given a supply of prednisone with proper dosing instructions to keep on hand to use during stressful situations with either medication choice (Norkus, 2012).
Conclusion
Understanding the ‘great pretender’ Addison's disease will help the pet to receive a quick diagnosis and treatment. When a patient presents in an Addisonian crisis it is imperative the team understands the treatment and care needed for the patient. Owners often need good communication by the veterinary team to understand the disease and how they will care for their pet at home. Being able to communicate with the client effectively will allow for the pet's best chance of long-term survival of this disease.

