Any object that enters sterile tissue or the vascular system is defined as a ‘critical item’. Critical items are so called due to the high risk of infection if such an item is contaminated with any microorganism, including bacterial spores (Rutala and Weber, 1999).
Sterilisation renders items safe for contact with tissue and blood without transmission of infection as long as sterility is maintained (Phillips, 2004). Sterility is considered an absolute term — to put it simply, an item is either sterile or it is not. Vegetative organisms are relatively easy to destroy, however spores are resistant, and as such referenced as a benchmark for achieving sterility (Gasson, 2009).
Disinfection of surgical instruments and equipment by boiling was introduced in the 1880s, replacing Joseph Lister's method of soaking items in carbolic acid (a phenol compound), and by 1876, heat-resistant bacteria were demonstrated. Around 1886, Ernst von Bergmann and his associates introduced the steam steriliser, but surgeons soon discovered that steam in itself was inadequate for sterilisation; steam must be under pressure in order to raise the temperature sufficiently to kill heat resistant microorganisms (Phillips, 2004). This basic concept of heating water in a closed vessel to produce steam above atmospheric pressure, thus at a higher temperature, has changed remarkably little since (Gasson, 2009). The science of sterilisation however, has moved on considerably. Modern day sterilisation processes must be able to demonstrate a minimum sterility assurance level (SAL) of 106. A SAL of 106 represents a one in a million chance of an organism surviving on an item and is universally considered as an acceptable level of risk (Gasson, 2009; Veerabadran and Parkinson, 2010).
Sterility of items in a veterinary practice may be achieved via a variety of methods, however steam under pressure (autoclave) is considered the most popular form and will be discussed here.
It is well recognised that to be effective, sterilisation requires time, contact temperature and, with steam sterilisation, high pressure. The effectiveness of sterilisation is also highly dependent on a further four factors:
- The type of microorganism — certain microorganisms are difficult to destroy. Bacterial spores are the most resistant of all living organisms because of their capacity to withstand external destructive agents. Spores of Geobacillus stearothermophilus are considered to be heat and moisture resistant organisms, thus are normally used in the validation and testing of steam sterilisation (Gasson, 2009).
- Bioburden — this is the relative number of actual or suspected microorganisms that may be found on a specific item or in the environment at a specific time. For sterilisation to be effective, the bioburden on items must be relatively low (Phillips, 2004).
- The amount and type of organic material remaining on an instrument — all instruments must be thoroughly cleaned prior to sterilisation. Blood or tissue remaining on poorly cleaned instruments acts as a shield to microorganisms during the sterilisation process.
- Design and care of surgical instruments — micro-organisms collect in, and are protected by, scratches, cracks and crevices such as the serrated jaws of haemostats. Thorough cleaning and regular inspection of surgical instruments is therefore essential. Ultrasonic cleaners can be useful for cleaning hard-to-reach areas.
Troubleshooting
Human error is cited as the main cause of steam sterilisation process failures, followed by equipment mal-function and steam quality/performance (Figure 1) (Chandrapati, 2008).
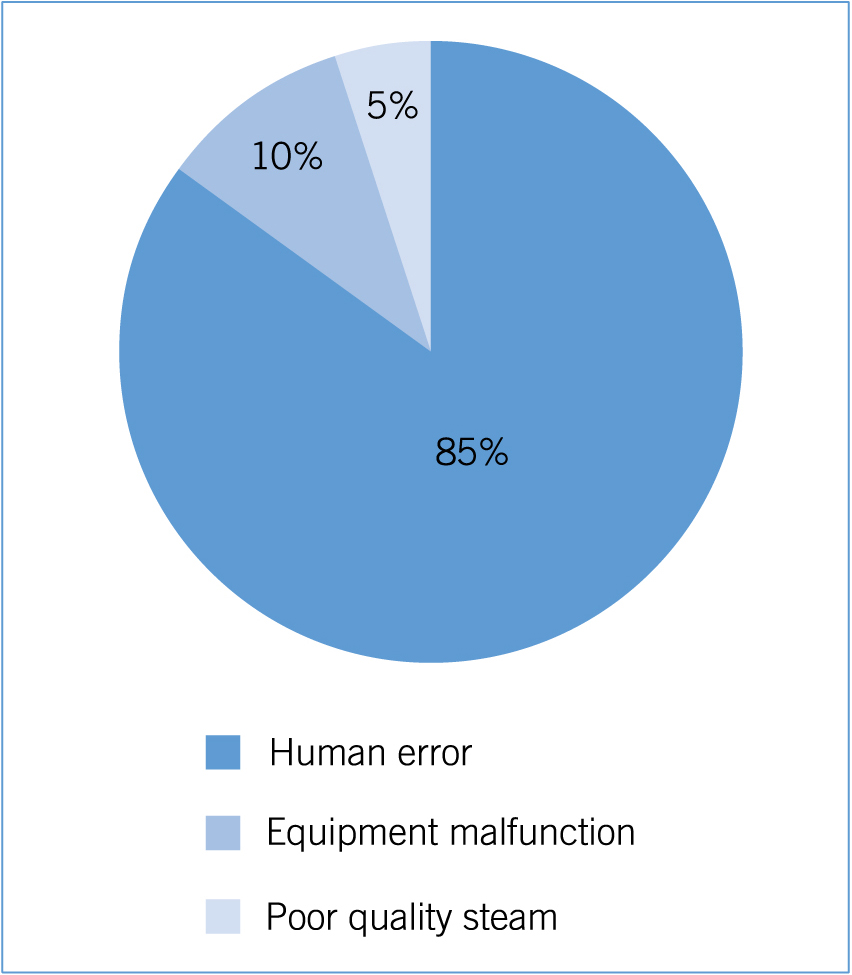
Human error
Correct loading of the autoclave: the type of auto-clave used dictates the type of load that may be safely processed within it. To achieve sterilisation, the sterilant, in the case of an autoclave saturated steam, must be able to fully penetrate the load. Improper loading patterns and overloading are frequently encountered problems. Correct loading of the autoclave is a critical step in the sterilisation process; the chamber of the autoclave must not be overloaded. The outer wraps of the packs must not touch the inner surface of the chamber and ideally should be separated by 3–5 cm (Hamilton, 2012). Steam will be unable to penetrate the packs effectively if they are crammed too tightly within the chamber (Weinman and Jones, 2006).
Inappropriate wrapping materials may also be used which will hinder the sterilisation process. When selecting a wrapping material, it is essential to ensure the selected material will act as a barrier to microbes in order to prevent contamination of the items within the pack. In addition, for the sterilisation process to be effective, the material must be sufficiently permeable to allow the sterilant to reach the contents within and must not react with the sterilisation agent, as this will compromise the process (Hamilton, 2012). Advantages and disadvantages of packing materials compatible with steam sterilisation are shown in Table 1.
Table 1. Packing materials compatible with steam sterilisation
| Wrapping material | Advantages | Disadvantages |
|---|---|---|
| Cotton muslin | Reusable; easy to handle | Requires double wrapping, not waterproof |
| Paper | Economical | Requires double wrapping; not waterproof |
| Polypropylene fabric | Durable; resistant to damage | Single use; requires double wrapping |
| Paper/newer materials such as Tyvek | Water-resistant; single wrap; long shelf life | Instruments may pierce the pouch |
from Hamilton (2012)
Equipment malfunction/ maintenance
In order to remain in optimum condition, autoclaves need to be used and maintained as per the manufacturer's instructions. Routine maintenance includes such things as cleaning of gaskets, chambers and external surfaces. The inside of the chamber must only be cleaned with nonabrasive products specified by the manufacturer. Door gaskets should be inspected for signs of wear, defects or deterioration and then wiped over with a damp, non-linting cloth. Inspection of the inlet and outlet valves is also essential to ensure these are not blocked; this would limit the effectiveness of steam penetration. It is essential to ensure the machine is cooled prior to performing any type of cleaning or maintenance. For optimum steam quality and in order to preserve the heating element of the machine, the use of distilled water rather than tap water is advocated within bench top autoclaves to prevent the build up of hard-water deposits. Tap water may be heavily contaminated with dissolved substances that can damage not only the machine itself, but can also redeposit bioburden on to the items to be sterilised (Gasson, 2009).
Regular servicing and inspection should be undertaken (in accordance with manufacturer's recommendations) by a qualified service technician. This will include calibration of the machine, which includes checking the pressure–sensing and temperature-sensing gauges, cycle timers, controls and recording devices. Proper calibration is essential for effective and reliable sterilisation (Caveney, 2012). Documentation regarding such information as the maintenance, date of servicing and parts replaced should be kept. In order to provide evidence that the sterilisation parameters have been met, it is good practice to keep records of the date, load, mechanical controls, chemical indicator and/or biological indicator from all processed loads.
Steam performance
The definition of autoclave means self closing, which refers to the fact that during the process the chamber door is prevented from opening due to high pressure inside the chamber (Hamilton, 2012).
It is beyond the scope of this article to discuss all the different types of autoclave available, however it is important to note that not all autoclaves are the same. They vary in design and performance, and you do indeed get what you pay for. The decision to purchase an autoclave based on cost alone is a false economy (Gasson, 2009). Knowledge of the types available can assist in making an appropriate purchase, hence research of different machines is advisable. Two com-mon types of autoclave, the gravity-displacement and pre-vacuum machines will be discussed here.
Gravity-displacement machines
Performance varies according to how air is removed from the chamber. Gravity-displacement autoclaves work on the principle that steam is lighter than air. Steam enters the top of the inner sterilising chamber from the narrow outer chamber and displaces heavier air downwards. The air is released via a thermostatic valve at the bottom of the chamber and released into the environment. Steam continues to be introduced under pressure and as more air is removed and the pressure continues to rise, the temperature also continues to increase (Hamilton, 2012).
For successful sterilisation in an autoclave, air must be completely replaced by steam. Hamilton (2012) reported one of the major problems with gravity-displacement autoclaves as air becoming trapped within hollow objects. Residual air within the sterilisation chamber hinders the optimal diffusion of steam. In the absence of air, steam condenses on coming into contact with a cooler object and thus effectively transfers heat and inactivates any microbial bioburden (Chandrapati, 2008). When residual air remains within the sterilisation chamber, this inhibits the condensation of steam on to the surfaces of the articles to be sterilised. The air is then compressed into the centre of the load, creating ‘cold spots’ (Gasson, 2009). Such air pockets are impermeable to steam, thus greatly reducing the efficacy of the sterilisation process (Chandrapati, 2008). Gravity-displacement autoclaves are considered the most basic of machines (Gasson, 2009) and while once commonplace within veterinary practice, they are becoming increasingly replaced by pre-vacuum machines.
Pre-vacuum sterilisers
Pre-vacuum sterilisers work by actively removing air from the sterilising chamber prior to the induction of steam. Creating a vacuum in the inner chamber results in the steam penetrating the entire chamber both quickly and more effectively than in a gravity-displacement system. There is no need for the air to be displaced, speeding up the process, and there are fewer issues surrounding air pockets being trapped within the chamber (Hamilton, 2012).
Monitoring devices
Monitoring devices known as chemical indicators (CIs) are designed to respond with a physical or chemical change to one or more of the physical conditions within the sterilisation chamber. They are used to detect sterilisation failures that may arise due to human error or equipment malfunction. There are six classes of CIs with Class 1 indicators the most basic, reacting only to the temperature component of steam sterilisation (Figure 2). These are often located on the outside of a pack to show that the pack has been exposed to a sterilisation process

Class 5 and Class 6 chemical indicators are considered the most sophisticated as they react to all critical parameters and should be considered for routine use within all packs (Figure 3) (Gasson, 2009).
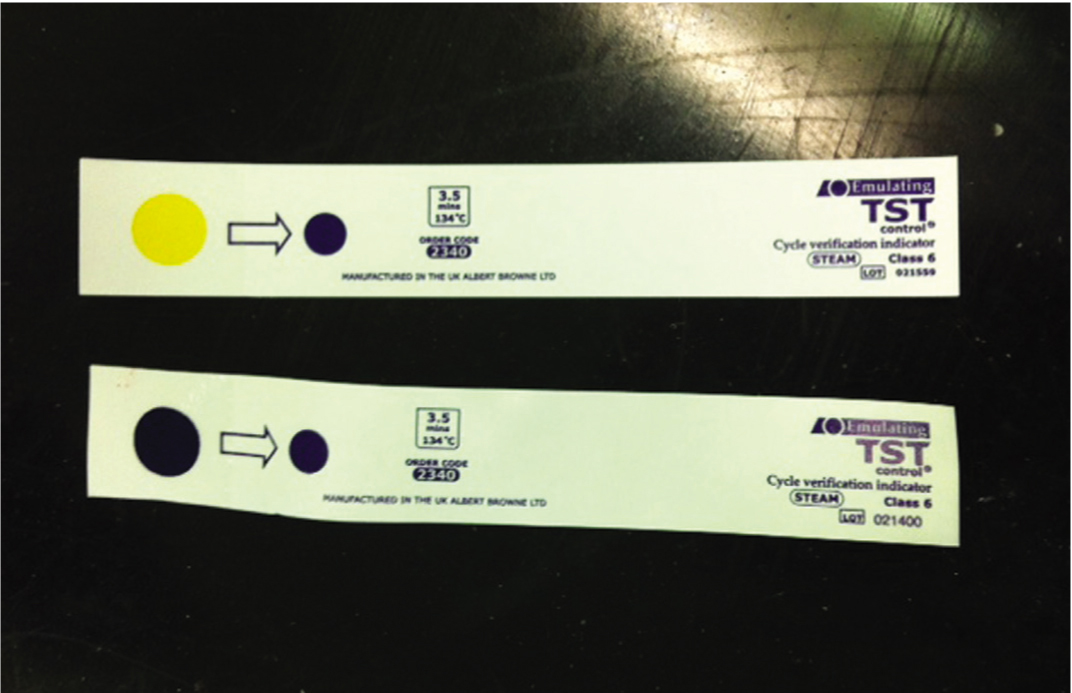
It must be noted however that a positive indicator change does not ensure the sterility of an item. A pass merely indicates the conditions at the test strip location were sufficient to bring about a change (Gasson 2009; Hamilton, 2012). To mean anything useful therefore, chemical indicators should be positioned in the most inaccessible part of the load. This is generally considered to be in the centre of the pack, as residual air may compress here during steam admission (Figure 4) (Gasson, 2009).

Biological indicators (BIs) involve the use of live bacterial spores contained in a glass vial or within a paper strip, and are generally considered the highest level of sterility assurance (Caveney, 2012). A number of different organisms are used for different protocols, however the bacteria chosen for use in BIs need to be non-pathogenic, spore-forming and many times more resistant to the sterilisation method than the most likely naturally occurring contaminants (Hamilton, 2012). The major disadvantage with BIs is that the results are not available immediately; once recovered from the load they require incubating for between 24–48 hours. For this reason they should be included within loads periodically, ideally at least once per week as part of an overall regimen to ensure that high standards of sterility and asepsis are maintained at all times (Hamilton, 2012). It is suggested however that BIs are included in all loads containing implants, with such loads being quarantined until the results of the BI testing are available (Association for Professionals in Infection Control, 2010). The importance of this is supported by a number of studies including Schneider et al (2005), which highlighted the superiority of BIs in being able to detect sterilisation failures that were frequently missed by other monitors. The study demonstrated failures, including incomplete air removal, were missed by a number of Class 5 and 6 CIs which displayed a pass result. Only the BIs consistently detected the suboptimal steam sterilisation failures.
Storage
Absolute storage times for sterile supplies are difficult to determine as they vary with the porosity of the wrapping material and the overall storage conditions. Sterilisation is a process, not a single event, and within the NHS event-related sterility is gaining in popularity compared with the more traditional time-related shelf life. Event-related sterility is the term that refers to the maintenance of the sterility of the packages until they are used. This is based on the concept that contamination of a sterile item is event related, and the probability of its occurrence increases over time and with prolonged handling, storage or environmental conditions (Association for Professionals in Infection Control, 2010). Event-related factors that contribute to the contamination of a package include air movement, traffic through the area, location, humidity, storage area, space and temperature. As a general rule packs should not be stored under sinks or in other locations where they may become wet. Closed or covered cabinets are favoured over open shelving, and in order to allow for adequate circulation, packs should be stored raised off the floor, below ceiling height and not directly touching the sides of containers or walls. The packaging material, chemical indicator and package integrity should be examined closely prior to use.
Wet packs
All sterilisation methods in which humidity, generally steam, is a parameter of the process potentially present the hazard of producing wet packs. Micro-organisms migrate easily through moisture when a pathway is provided from outside to inside a package (Phillips, 2004). Water droplets may be visible on the inside or outside or absorbed moisture may be seen or felt. A pack should be considered unsterile and unacceptable for use if it is wet. This rule must also be applied to stained packages as this may indicate moisture was present and has subsequently dried (Figure 5). Drying items after removal from the autoclave is not acceptable (Gasson, 2009), the cause of wet packs must be investigated promptly and rectified.
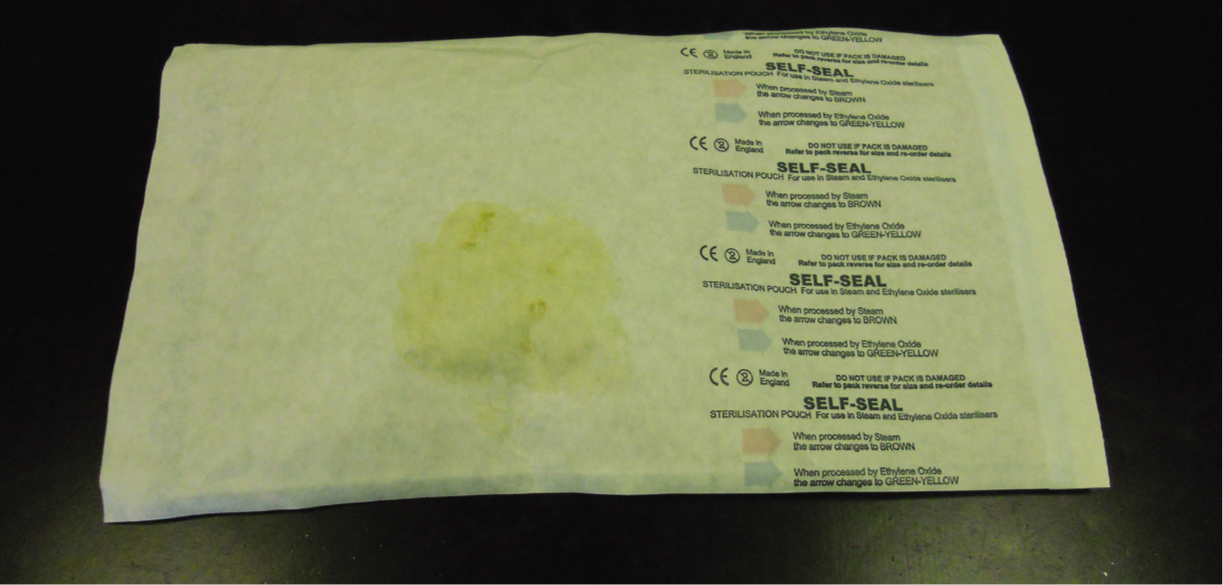
This fact was reinforced by Dancer et al's (2012) retrospective study which investigated the sudden increase of surgical site infections following orthopaedic and ophthalmic surgeries within a single NHS hospital. An ‘outbreak investigation committee’ (OIC) conducted extensive audit and screening exercises of issues such as hand hygiene compliance, operating theatre practices, clinical teams, theatre lists and ventilation systems, in an attempt to determine the cause. Systematic analyses of all the variables failed to identify any common areas, issues or individuals that could be linked to the patient cohort under investigation. Environmental, hand hygiene and screening audits were also satisfactory. Thea-tre staff at the hospital eventually voiced their concerns over the number of damp or stained packages containing surgical instruments that were being received from the instrument sterile services provider. An investigation was immediately launched and 20 surgical packs were chosen for sampling, ten of which had been identified as wet and/or stained by theatre staff. Eight of the visually contaminated packs sampled grew Bacillus spp. and/or skin flora including coagulase-negative staphylococci from inner wrappings and/or instruments. Since investigations failed to identify any other cause for the outbreak, the sudden increase in surgical site infections was attributed to poor handling practices both within the sterilisation plant and by theatre staff. The OIC hypothesised that given the nature of the staining on the packs, instrument sets were dampened, either from excess moisture in the autoclave or condensate inside the packages subjected to rapid cooling. This would have created a portal of entry for skin and environmental flora during transfer and handling by staff. This situation could be extrapolated to veterinary practice and reinforces the need for visually examining instrument packs for wetness and/or staining prior to use.
Conclusion
If used correctly, sterilisation by steam is a rapid, cost-effective, safe and effective means to render heat-stable items free of microbial contamination. Sterilising items for use in surgery, however, requires much more than just knowledge of how to operate the autoclave. As microorganisms are an invisible enemy, inspection alone cannot be relied on to provide assurance of sterility. As human error is frequently associated with sterilisation failures, it cannot be assumed that just because an item has completed a cycle within the autoclave, this can confirm sterility either.
In order to ensure sterility certain criteria need to be met, monitored and documented. Staff training and written protocols must be established for all phases of the sterilisation process. Such protocols must be read, understood, followed and updated regularly in the light of new technologies.
Key Points
- Human errors are frequently associated with sterilisation failure
- Sterility is an absolute term.
- Blood or tissue remaining on poorly cleaned instruments acts as a shield to micro-organisms.
- Inappropriate wrapping materials will hinder the sterilisation process.
- A positive result from a monitoring device does not ensure sterility of an item.
- Microorganisms migrate easily through moisture when a pathway is provided from outside to inside a package.
To answer the CPD questions on this article visit www.theveterinarynurse.com

