The term neoplasia is related to a group of conditions caused by the abnormal proliferation of cells often leading to the development of a tumour (Argyle et al, 2009). Loss of the in-built growth-limiting mechanisms within a cell is often caused by mutations at the DNA level. This is known as carcinogenesis (Pitot, 1993; Pinho et al, 2012).
On a cytological level, there are three main categories of neoplasm as detailed in Table 1 (Baba and Câtoi, 2007; Argyle et al, 2009):
- Epithelial
- Mesenchymal
- Round cell tumours.
Table 1. Categories of neoplasm
| Benign | Malignant | |
|---|---|---|
| Epithelial: glandular, squamous or transitional | Adenoma: glandular; e.g mammary or sweat gland | Squamous cell carcinoma; e.g skinTransitional cell carcinoma; e.g bladder |
| Mesenchymal: fat, cartilage, fibrous tissue | Lipoma; fatty tissue Chondroma; cartilage tissue | Fibrosarcoma; e.g oral tumour in dogs |
| Round cell: majority are of haemolymphatic origin e.g mast cell, histiocyte, plasmacyte, lymphocyte | Histiocytoma | LymphomaMast cell tumour Malignant histiocytosis |
These cellular origins can allow us to predict the way a tumour is likely to behave locally and determine the like-lihood of spread as not all neoplasms are malignant. If a neoplasm is benign, it is likely to be slow-growing, well-differentiated and of no clinical concern to the patient unless it is a space-occupying lesion, e.g a brain tumour. If a neoplasm is malignant, it is known as cancer and has the potential to spread from the primary source to other areas in the body (Argyle et al, 2009).
Assessing cancer spread
Assessing the spread, or metastasis, of a primary malignant neoplasm is known as staging. This often involves sampling or removing the drainage lymph node adjacent to the affected site in combination with screening the main body cavities. In order to determine the extent of primary and secondary tumours, systems such as the tumour-node-metastasis (TNM), developed by the World Health Organization (WHO) in 1980 are used internationally in both humans and animals. Alongside grading — that is, the histopathological assessment of whole or sections of neoplastic tissue obtained by biopsy — these systems allow for correlation between primary tumour size and metastasis, and aid in the prediction of prognosis (Gundim et al, 2016).
What is the most common neoplasia in dogs and cats?
When estimating the incidence of neoplasms in the canine and feline population, we must consider the main variables of age, sex and breed (Dorn et al, 1968; Withrow et al, 2012). Further variables involved include whether an animal is purebred, entire or over expected bodyweight. For example, older, neutered, overweight animals are at highest risk of developing lipomas (Baioni et al, 2017; O'Neill et al, 2018).
Dogs
In 2004, the Kennel Club conducted a study to determine the greatest causes of mortality in purebred dogs. The study highlighted the highest incidence of cancer in breeds such as the Flat-Coat Retriever, Bernese Mountain Dog, Staffordshire Bull Terrier and Rottweiler (Adams et al, 2010). However, obtaining accurate information regarding prevalence is often hindered by confounding factors such as insurance, missed diagnoses, questionnaire participation and, indeed, presentation of the animal to the veterinary practice in the first place (Egenvall et al, 2009; Baioni et al, 2017). As a result of these factors, retrospective data collection is often used to measure prevalence (O'Neill et al, 2018). Although these studies are derived from practice databases, they lack the crucial integration between owner and practice that a prospective study can offer. This integration, if well designed, allows for the collection of more superior data and, in turn, can yield a more accurate representation of prevalence and incidence. However, potential for selective reporting and the Hawthorne effect must be considered (Rose et al, 2016).
Irrespective of study type, there are various reports estimating the prevalence of neoplasms in dogs and cats. Overall, taking age and sex into consideration, most studies agree on mammary tumours as the most common neoplasm in female dogs. In a study by Merlo et al (2008), looking at the occurrence of spontaneous tumours in a population of dogs in Genoa, Italy, mammary tumours accounted for nearly 70% of 7000 biopsy specimens over 17 years. The age bracket for females most at risk was found to be 10 years old onwards (Merlo et al, 2008).
In many studies, the haemopoietic cancer lymphoma (lymphosarcoma) follows in close succession in both male and female dogs. Lymphoma can manifest in many body systems; however, in the dog, multicentric lymphoma accounts for up to 80% of cases (Kang et al, 2019). It affects the lymph nodes on both the cranial and caudal halves of the body and readily spreads to the spleen and bone marrow in stages 4 and 5 of the disease respectively.
Skin tumours are also among the most common presentations in first-opinion practice. These range from highly malignant mast-cell tumours to benign lipomas and histocytomas, the latter often spontaneously regressing.
Cats
Cat neoplastic incidence is generally lower than in dogs (Withrow et al, 2012). However, the proportion of malignant tumours tends to be higher. In a study by Vascellari et al (2009), it was found that malignant tumours had nearly a five-fold greater incidence than benign tumours. This ratio is similar to that observed when considering feline mammary tumours alone (Morris, 2013). Lymphoma, squamous-cell carcinomas, mammary carcinomas and injection-site sarcomas dominate neoplasia discussion in cats. Squamous-cell carcinomas account for approximately 80% of oral tumour presentations (Cannon, 2015). They are highly invasive and often cause a significant amount of local trauma. Secondary infection of the area and metastasis to the lungs are common findings. Increased risk factors have been identified such as exposure to tobacco smoke and flea collars but these associations are not usually made with individual patients (Bertone et al, 2003).
In the 1970s feline leukaemia virus (FeLV) was important in understanding the pathogenesis of lymphoma in cats (Francis et al, 1979; Hartmann, 2012; Beatty, 2014). Better preventative healthcare means this is now much less of a factor; however, it has been shown that lymphoma incidence in cats is still on the rise (Louwerens et al, 2005). With a wider variety of common lymphoma presentations in cats (for example, mediastinal and low-grade gastrointestinal), other aetiologies must now be explored. Inflammatory bowel disease is a significant focus when considering low-grade gastrointestinal lymphoma and distinguishing between the two often proves to be difficult (Paulin et al, 2018). The role and mechanism of feline immunodeficiency virus in the manifestation of feline lymphoma is still being established (Beatty, 2014).
Injection-site sarcomas (fibrosarcoma) occur in approximately 1 in 10 000 vaccinated cats and are a subject of debate (Zabielska-Koczywąs et al, 2017). Although the pathogenesis is unclear, chronic inflammation resulting from repeated injections in the interscapular area is thought to play an important role in the development of these highly invasive and malignant tumours of mesenchymal origin (Hartmann et al, 2015).
Minimising subcutaneous injections in hospitalised feline patients is now encouraged along with reducing the use, where possible, of vaccines containing adjuvant. These include rabies and FeLV (Martin, 2003; Zabielska-Koczywąs et al, 2017). Using alternative vaccine locations has also been suggested as a way to aid earlier detection of a mass, as earlier recognition and treatment can affect prognosis significantly (Saba, 2017).
What is involved in making a diagnosis?
There are several factors to consider in distinguishing a primary tumour from distant metastasis:
- Physical examination: allows assessment of the primary tumour size and location. Assessment of attachment of the tumour to underlying structures may allow for prediction of surgical possibility. Regardless of how obvious the primary tumour is, a thorough physical examination should always be performed. For example, palpation of a mammary tumour alongside presentation of a bleeding abdominal tumour may result in additional surgery or the decision to avoid surgery entirely. Assessment of the usual parameters such as mucous membranes allows for recognition of potential secondary complications such as anaemia and hypoxia
- Primary diagnostics: bloods — haematology, biochemistry, electrolytes, C-reactive protein (indication of large-scale or widespread inflammation in dogs); fine-needle aspirate where appropriate; radiography and ultrasound. If the animal presents with fluid in a body cavity, thoraco/abdominocentesis can be used alongside cytology to aid in making a diagnosis. Note: some solid tumours cause fluid to build up but do not always shed cells into the fluid that could result in a diagnosis
- Secondary diagnostics: more invasive biopsy, bone marrow biopsy, endoscopy, bronchoscopy, magnetic resonance imaging (MRI) (brain and spinal cord), computed tomography (CT scan) (soft tissue and assessment of metastasis). Depending on practice facilities and expertise, it may be more appropriate to refer animals if these are required.
As mentioned, cytological interpretation of tumours alone can sometimes yield a diagnosis. However, poor aspiration of cells with fine-needle aspirates can yield false-negative results, especially if the site is not easily accessible. Aspiration of inflammatory areas around the neoplasia where cells are not normal but not specifically showing characteristics of malignancy can also lead to an inconclusive result.
It can be particularly difficult to distinguish between neoplastic and inflammatory cells in cats. Owner frustration in this area is not uncommon, especially if costs are a concern, so warning them prior to sampling is important. Biopsy of a tissue, whether it be a punch, guillotine or excisional, allows for greater efficacy in obtaining a diagnosis. However, impact on future surgical removal must be considered when taking biopsies.
Differential diagnoses are essential as suspected tumour type can influence the form of biopsy taken. Where invasiveness is considered minimal and enough healthy skin is present around the tumour tissue, excisional biopsy is an option. This allows for the assessment of clear margins and prediction of recurrence. However, if margins are incomplete, a second surgery may be necessary. All of this must be discussed in detail with the owner beforehand.
It is also important to convey that surgical techniques have their drawbacks. Requirement for sedation or anaesthesia must be considered alongside the potential for postoperative complications such as swelling, haemorrhage and pain. This is why when presented with a suspected lipoma that is causing no harm to animal in terms of size, location or inflammation, it is perfectly acceptable to agree with the client to monitor. Investing in callipers and designing a body map that can be kept and amended for individual patients is a good way of assuring owners that the masses are being actively monitored during their visits.
What are the most common treatments?
When deciding on an appropriate treatment course for an animal, there are two fundamental considerations:
- Suitability of the animal for the treatment plan
- Suitability of the treatment plan for the owner.
The aim of the treatment must be made clear to the owner: is the treatment palliative or definitive? The purpose of a palliative treatment in some cases is to alleviate pain and signs associated with the tumour; whereas in others, it is to slow down cancer progression. For example, in a dog with a highly malignant osteosarcoma in the hind leg, there is a high chance that there is already micro-metastasis within the lungs even if not visible on radiography; therefore, any treatment is not definitive. These bone tumours are often very painful and, in some dogs (older large-breed dogs with concurrent osteoarthritis), it is not appropriate to amputate the leg. In these cases, palliative radiation therapy is an option to aid the alleviation of pain instead.
Steroids are also frequently used as a palliative treatment option; for example, in cases of lymphoma where lymphnode enlargement is causing dyspnoea, prednisolone can significantly reduce the size of the lymph nodes for a short amount of time. This is in comparison to a full chemotherapy protocol where the aim of treatment is to have the same effect but while promoting longer-term survival. Mean survival times (MSTs) vary. Even though the animal may go into remission, chemotherapy is still considered a palliative option. The cases where a definitive treatment option is available often involve a non-malignant neoplasm that can be surgically removed with adequate margins.
As outlined in Table 2, there are many considerations within each treatment category. Figure 1 demonstrates the personal protective equipment recommended when administering chemotherapy both for administrator and other personnel in the designated area. It is worth noting that gloves should be powder-free and eye and respiratory protection is essential in case of spillage. Ready-made chemotherapy packages (e.g from ChemoPet) come in oral or intravenous formulations. If intravenous administration is required the package comes with a specific safety t-connector (Figure 2), sterile saline flushing solution and a bandage for the patient after catheter removal. All materials used in the administration of chemotherapy must be disposed of appropriately in cytotoxic-waste bins. These can be ordered from the chemotherapy provider. Oral chemotherapy can be administered at the practice or at home; however, this may not be appropriate for all owners. A thorough conversation must be had with the owners about cytotoxic risk and understanding assessed to determine suitability. For example, the risk is considered greater if the administrator is pregnant or immunosuppressed. Owners must adhere to personal protective equipment instructions and avoid direct handling of their animal's excreta and saliva. Ordering from an external source eliminates the requirement for fume cupboards and extractor fans during the preparation process. The chemotherapy comes with detailed instructions and can be carried out by an experienced registered veterinary nurse (RVN). All of this makes chemotherapy a perfectly feasible first-opinion option; however, further information should be sought if offering and administering chemotherapy is being considered. Equally, if a practice wishes not to administer chemotherapy, referral to an oncology centre is always an option. Useful resources include The American College of Veterinary Internal Medicine (ACVIM) small animal consensus statement on safe use of cytotoxic chemotherapeutics in veterinary practice, and guidelines and further information can be found on the Government Health and Safety Executive website (www.hse.gov.uk/coshh).

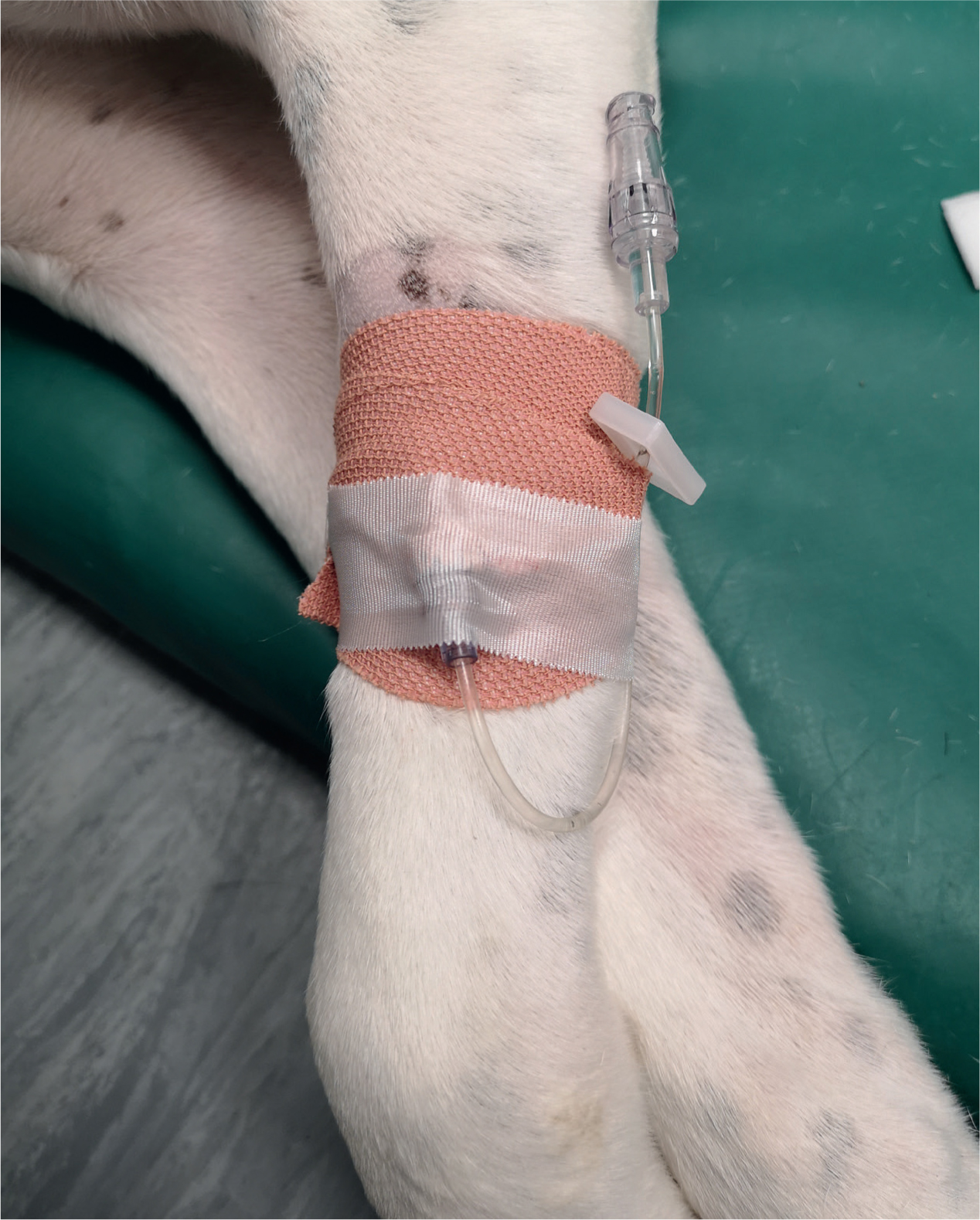
Table 2. Considerations for some of the main neoplasia treatment options
| Treatment plan | Considerations prior | Considerations during | Considerations post |
|---|---|---|---|
| Surgical |
|
|
|
| Chemotherapy |
|
|
|
| Radiotherapy |
|
|
|
| Palliative |
|
|
|
In a study by Williams et al (2017), it was found that 58% of questionnaire responders would not put their dog or cat through chemotherapy as a result of having had previous negative experiences with it. Although the administration of chemotherapy in companion animals does not cause widespread alopecia as it does in humans, the potential effects should not be underestimated. Overtly, side effects of vomiting, diarrhoea and subdued demeanour are common (MacDonald, 2009; Cunha et al, 2017). Specific chemotherapy toxicities also occur. Examples include liver (lomustine) and cardiac (doxorubicin) toxicity. Prior to and during the administration of doxorubicin intravenously, monitoring of the patient's electrocardiogram (ECG) is essential (Hallman et al, 2019). It is important that the owner is fully informed of these side effects and that they feel adequately supported when drawing correlations between what we hope the quality of life to be during chemotherapy and what it actually is. Immunosuppression is also a very common side effect that needs to be monitored carefully. Neutropenia is a common side effect and if the neutrophil count is less than 1.5×109 cells/litre during a protocol, the chemotherapy should be postponed even if the animal is non-pyrexic and non-symptomatic (MacDonald, 2009). Platelets also need to be carefully monitored. The evidence on the use of prophylactic antibiotics with chemotherapy protocols is conflicting but no matter the chemotherapy, failure to spot severe immunosuppression can quickly lead to sepsis (Bisson et al, 2018). Predisposition to infection in the veterinary practice, particularly in hospital environments, needs to be considered.
What is a paraneoplastic syndrome?
A paraneoplastic syndrome is a clinical sign induced by a tumour away from the primary site. These are often caused by hormones, hormone-like substances, cytokines and enzymes (Elliott, 2014). If left untreated, they can be life-threatening due to their potentially multisystemic effects. If recognised, they can be resolved and used as a biomarker for remission after appropriate tumour treatment (Finora, 2003). Table 3, adapted from Decision Making in Small Animal Oncology (Argyle et al, 2009), provides an overview of the most common paraneoplastic syndromes in animals.
Table 3. Most common paraneoplastic syndromes in animals
| Paraneoplastic syndrome | Tumours of origin | Clinical signs | Other possible causes | Specific treatment |
|---|---|---|---|---|
| Hypercalcaemia (raised total and ionised calcium) | Anal sac adenocarcinoma Lymphoma and leukaemia Other carcinomas; mammary, thyroid | Polyuria and polydipsia Weakness Dysrhythmias Vomiting and diarrhoea | Renal failure, addisons, bone disease, toxins and lab error (haemolysis) | Isotonic fluid therapy Use of furosemide concurrently with Intravenous fluid therapy (IVFT) — monitor kidneys and supplement K+ if required |
| Hyperglobulinaemia | Multiple myeloma (bone marrow neoplasia) Lymphoma NB: Electrophoresis used to distinguish between these | Hyperviscosity of the blood can have multisystemic effects | Feline infectious peritonitis in catsChronic immunologic diseases | Treatment of the primary tumour or referral treatment such as plasmapheresis (autotransfusion with the removal of plasma) |
| Hypoglycaemia | Insulinoma (pancreatic neoplasia)Other carcinomas, e.g. hepatic | Polyphagia, weakness, muscle tremors and seizures | Sepsis, liver failure, poorly controlled diabetes | Careful when feeding; high-sugar diet can promote the release of insulin, worsening hypoglycaemiaPrednisolone — causes insulin resistanceSurgery is treatment of choice |
| Tissue oedema and gastric ulceration | Mast cell tumour; tends to be cutaneous in dogs, visceral in cats | Oedema and erythema of skinHypotension or spontaneous bleeding (due to heparin release from mast cells) | Allergic reaction, bites, previous surgery (oedema) Non-steroidal anti-inflammatory drug (NSAID) use (gastric ulceration) | Chlorphenamine (cutaneous) Ranitidine and famotidine (visceral) |
| Cachexia | All in end stages | Severe muscle wasting and weight loss | Other diseases affecting metabolism, e.g. end-stage kidney disease | Accurate nutrition calculations; diet, enteral feedingPrednisolone stimulates hunger |
| Anaemia | Potentially all tumours; either anaemia of chronic disease or haemolytic anaemia from haemopoietic neoplasia (slide agglutination/Coomb's) | Lethargy, weaknessJaundice if haemolytic | Anaemia of chronic diseaseSlow, low grade bleeding | Packed cell volume (PCV) checks alongside tumour specific therapy and sometimes immunosuppressors if haemolytic origin |
Fundamental aspects of quality of life
Quality of life assessment should focus on an animal's physical, psychological and social functioning (Haes, 1996; Yeates and Main, 2009). Over the course of a career, veterinary surgeons put to sleep many patients where cancer has taken over their bodies causing poor quality of life. However, end-stage primary renal disease, heart disease or osteoarthritis are also frequent causes of pain and discomfort, despite best treatment efforts, and result in euthanasia when quality of life is no longer sufficient.
There tends to be a lot of stigma around animal oncology when considering the question to treat or not to treat. However, like any disease, treatment choice should be based on the attempt to strike a balance between quality and quantity of life. No matter the disease, nor the evergrowing advancements and treatment options, our duty of care to make the right decision for our animals andd clients remains the same. For more detailed discussion on quality of life, please consider the appropriate research in the further reading section below.
Conclusion
Recognising neoplasia in pets is part of every-day practice in the veterinary world. There are many options available to diagnose and treat our patients but ultimately, our primary responsibility is to guide owners in making the right decision for their animal's quality of life.
KEY POINTS
- Not all neoplasms are malignant.
- Tumours/neoplasms arise from three main cellular origins.
- A thorough clinical examination is imperative regardless of the tumour.
- Paraneoplastic syndromes can be the first indication of a tumour or used for monitoring remission.


