The pancreas is a lobulated organ that sits within the cranial abdomen, consisting of left and right lobes connected by a central body of tissue (Spielman, 2015). The pancreas is responsible for both endocrine (where substances are secreted directly into the bloodstream) and exocrine (where substances are secreted into a duct) functions. Endocrine functions of the pancreas involve islets of Langerhans cells which synthesise and release the hormones insulin and glucagon, involved in the regulation of blood sugar (Steiner, 2020). Surrounding these islet cells, and responsible for the exocrine function of the pancreas, are the acinar cells, which synthesise and secrete enzymes and zymogens involved in the digestion of food. Epithelial cells of the pancreatic ductules produce bicarbonate which acts as a buffer to the gastric acid in the duodenum (Gordon, 2011).
Pathophysiology
Pancreatitis is described as the most common exocrine disease affecting both canines and felines (Steiner, 2020). Premature activation of digestive enzymes results in pancreatic autodigestion, leading to pancreatitis. During episodes of pancreatitis, an apical block (secretory block) occurs, allowing zymogen and lysosomal granules to become co-localised within the acinar cell. The lysosomal protease cathepsin B activates trypsinogen, released from the zymogen granules, to trypsin. Production of trypsin continues until eventually the pancreatic secretory trypsin inhibitor (PSTI) is overwhelmed, at which point pancreatitis develops as the pancreatic digestive enzymes become activated within the acinar cell. In a normally functioning pancreas, both trypsinogen and cathepsin B would be released directly into the pancreatic duct (Mansfield, 2012).
Inflammation stimulates the movement of neutrophils into the acinar cell, vasodilation and increased permeability of local blood vessels. Neutrophils contain oxidising agents, known as reactive oxygen species (ROS), which are directly damaging to cells. Infiltration of neutrophils brings ROS, cytokines and nitric oxide into the acinar cell and further exacerbates inflammation (Mansfield, 2012). Nitric oxide (NO) is a molecular compound associated with providing protection to the pancreas, however, during cases of pancreatitis where pancreatic perfusion may be compromised, NO levels rise and become toxic, leading to cell necrosis (Mansfield, 2012).
Pancreatic microcirculation is of significance during pancreatitis. Mansfield (2012) describes disturbances in microcirculation including an increase in vascular permeability and microthrombi formation, leading to decreased pancreatic function. Hypovolaemia often develops in these patients, resulting in reduced splanchnic circulation and decreased gastrointestinal perfusion, which results in the development of complications on intestinal reperfusion, further exacerbating the inflammatory state. Other processes that perpetuate damage include; ‘cytokine storms’, where cytokines and chemokines become activated within the pancreas leading to further inflammation, the complement system and the renin-angiotensin-aldosterone system (Mansfield, 2012; Mansfield and Beths, 2015).
As well as the array of complex conditions caused by severe pancreatic inflammation, acute pancreatitis (AP) can also lead to the development of renal failure, acute lung injury, disseminated intravascular coagulopathy, chronic pancreatitis and exocrine pancreatic insufficiency (Mansfield, 2012).
Risk factors
AP is most commonly idiopathic but may occur secondary to a range of conditions including: toxin ingestion; dietary indiscretion; drug therapy; co-existing disease such as triaditis in felines; infectious diseases; trauma and surgery; hyperadrenocorticism and hyperlipidaemia (Gordon, 2011; Jensen and Chan, 2014).
Research has shown that there is a higher prevalence of pancreatitis in certain breeds, suggesting an inherited breed predisposition to the disease. Miniature schnauzers, terriers and miniature poodles are all over-represented for the condition (Jensen and Chan, 2014). Human literature reports that while alcoholism and gallstones remain the main contributors to the development of acute pancreatitis, there is also a definite genetic predisposition. One human study in children identified that between 5 and 8% of cases of acute pancreatitis were hereditary because of a mutation of one of several genes, including the PSTI gene (Ong and Fock, 2012; Bai et al, 2011). It is thought that the genetic predisposition causing miniature schnauzers to more commonly develop AP is also associated with the PSTI gene. This, alongside their tendency to develop hypertriglyceridaemia, results in their overrepresentation for the disease (UFAW, 2011).
Clinical signs and diagnosis
The two most commonly reported clinical signs present in patients with AP are vomiting and abdominal pain (Jensen and Chan, 2014). Other clinical signs that may be seen include: anorexia; diarrhoea; weakness and lethargy; dehydration and signs of circulatory shock (Gordon, 2011; Steiner, 2020) (Figure 1).
Bloodwork may reveal anaemia, azotaemia, neutrophilia with a left shift, thrombocytopenia, hypoalbuminaemia and elevated liver enzymes. The two most common electrolyte abnormalities associated with AP are hypocalcaemia and hypokalaemia (Gordon, 2011; Jensen and Chan, 2014) (Figure 2).

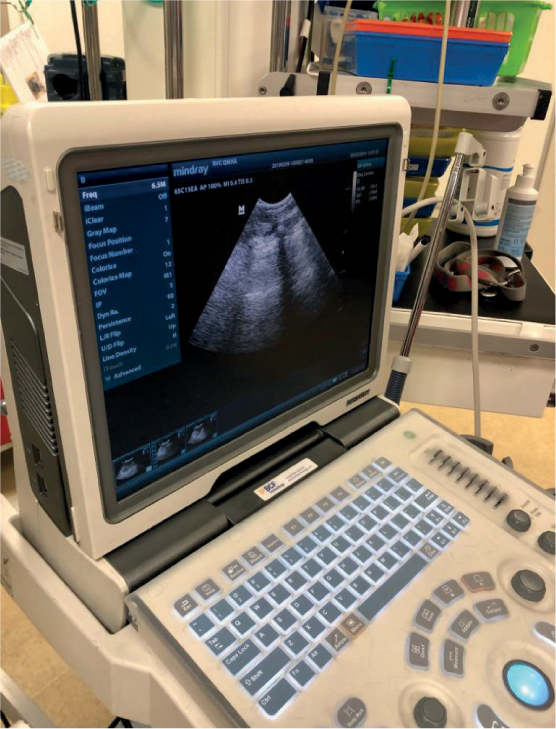
Radiographs are not commonly used to diagnose pancreatitis, but may be useful in ruling out other causes of vomiting and abdominal pain in these patients. If used, displacement of abdominal organs and a loss of serosal detail may be seen (Gordon, 2011). Ultrasonography is currently the most commonly used form of imaging for diagnosing AP in both canine and feline patients. An enlargement or irregularity of the pancreas, as well as areas of hypoechoic tissue, are significant changes associated with the presence of AP (Jensen and Chan, 2014) (Figure 3).
Pancreatitis may be present in either the acute or chronic form and distinguishing between the two can be challenging. A true diagnosis of either acute or chronic pancreatitis can only be achieved from examination of histological samples, since fine-needle aspirates (FNAs) may miss significant lesions present. FNAs may however be useful in diagnosing pancreatic necrosis and neoplasia (Gordon, 2011).
AP is typically associated with pancreatic oedema, pancreatic necrosis and a neutrophilic infiltrate, all of which are deemed to be reversible. Chronic pancreatitis is associated with permanent changes to the pancreatic parenchyma such as atrophy, fibrosis and a mononuclear inflammatory cell infiltrate (Watson, 2012). It is expected that in cases of AP, following treatment, the pancreas should return to normal both histologically and functionally, while in cases of chronic pancreatitis, the changes are often permanent, resulting in a loss of function which may progress to the development of other conditions such as diabetes mellitus or exocrine pancreatic insufficiency (Watson, 2012). Watson (2012) discussed the difficulty in diagnosing between acute and chronic pancreatitis since acute flare-ups of chronic disease often occur and may be mistaken for AP and similarly, recurrence of AP may be mistaken for chronic pancreatitis. Since many dogs will not have a pancreatic biopsy performed, diagnosis of the disease is usually based on clinical signs (Watson, 2012).
Pancreatic lipase testing
Historically serum lipase and amylase levels were used to diagnose pancreatitis, since levels are increased during inflammation, but both the sensitivity and specificity of these tests are poor for the diagnosis of pancreatitis as many other tissues also synthesise lipase and amylase (Suchodolski, 2013; Owens, 2019). It is now understood that pancreatic lipase only should be measured to increase the sensitivity for pancreatitis. The most widely accepted test for pancreatitis is pancreatic lipase immunoreactivity (PLI) which tests lipase of pancreatic origin only. Two forms of the test are available; SNAP PLI and Spec PLI, and a study carried out by Haworth et al (2014) found good agreement between both. Owens (2019) suggested that the specificity of the Spec cPL is higher so it should be considered as a follow-up test to a positive SNAP cPL for a definitive diagnosis.
Pancreatic scoring systems
In human medicine, it is considered vital to distinguish between those patients with mild and severe AP in order for appropriate treatment to be implemented to reduce mortality rate (Fabres et al, 2019). Two previously reported scoring systems for pancreatitis in canine patients have been suggested. The first system was based on assessing organ system dysfunction alongside elevated serum lipase or amylase levels, however, this was suggested before the production of specific lipase immunoassays. The second system assessed clinical severity against system dysfunction of four major body systems: cardiac and respiratory, intestinal integrity and vascular force, however neither scoring system have been validated (Fabres et al, 2019).
Fabres et al (2019) created two validated scoring systems known as the canine acute pancreatitis severity score (CAPS) and simplified CAPS for a quicker determination of severity. Four risk factors for short-term death of canine patients with AP were identified: systemic inflammatory response syndrome (SIRS), coagulation disorders, hypocalcaemia and increased creatinine. Use of the CAPS scoring allows for a more accurate prediction of outcome based on severity scoring and therefore quicker initiation of appropriate treatments.
Treatment
Treatment for AP is mainly supportive, often involving the administration of fluid therapy, antiemetics, gastroprotectants, nutrition and analgesia (Gordon, 2011). Nutrition and analgesia are extremely important in the management of these cases and will be discussed in greater detail later in this article (Figure 4).
The aim of fluid therapy is to correct acid–base disturbances, correct electrolyte abnormalities and maintain tissue perfusion. Mushtaq et al (2017) discussed the theoretical benefit of using an alkalinising fluid to increase the pH and prevent further trypsin activation within the acinar cell.
The administration of fresh frozen plasma (FFP) transfusions during pancreatitis is controversial with one recent study demonstrating a higher mortality rate in those canine patients with AP that were administered FFP (Cridge and Sullivant, 2018). While Cridge and Sullivant (2018) suggested that plasma use should be reserved for those patients with coagulopathies only, Mushtaq et al (2017) described that the anti-inflammatory proteins such as albumin found in FFP could be beneficial in providing oncotic support in AP patients.
Antiemetics are a mainstay in the treatment of AP and should be used to treat vomiting and nausea. Mushtaq et al (2017) explained vomiting in AP patients is centrally and peripherally mediated so a suitable antiemetic such as maropitant should be used as it blocks both the neurokinin 1 receptor and substance P production associated with centrally and peripherally-mediated emesis respectively. Mansfield and Beths (2015) suggested that even patients not showing signs of nausea should receive antiemetics to encourage nutritional intake earlier during hospitalisation.
Mushtaq et al (2017) further discussed the use of proton pump inhibitors to increase gastric pH and suggested there is some evidence that this reduces exocrine pancreatic stimulation. Proton pump inhibitors and H2–receptor antagonists are considered useful in the treatment of patients with AP, as they may help to reduce the risk of gastric ulceration and oesophagitis (Cridge and Sullivant, 2018) (Figure 5).
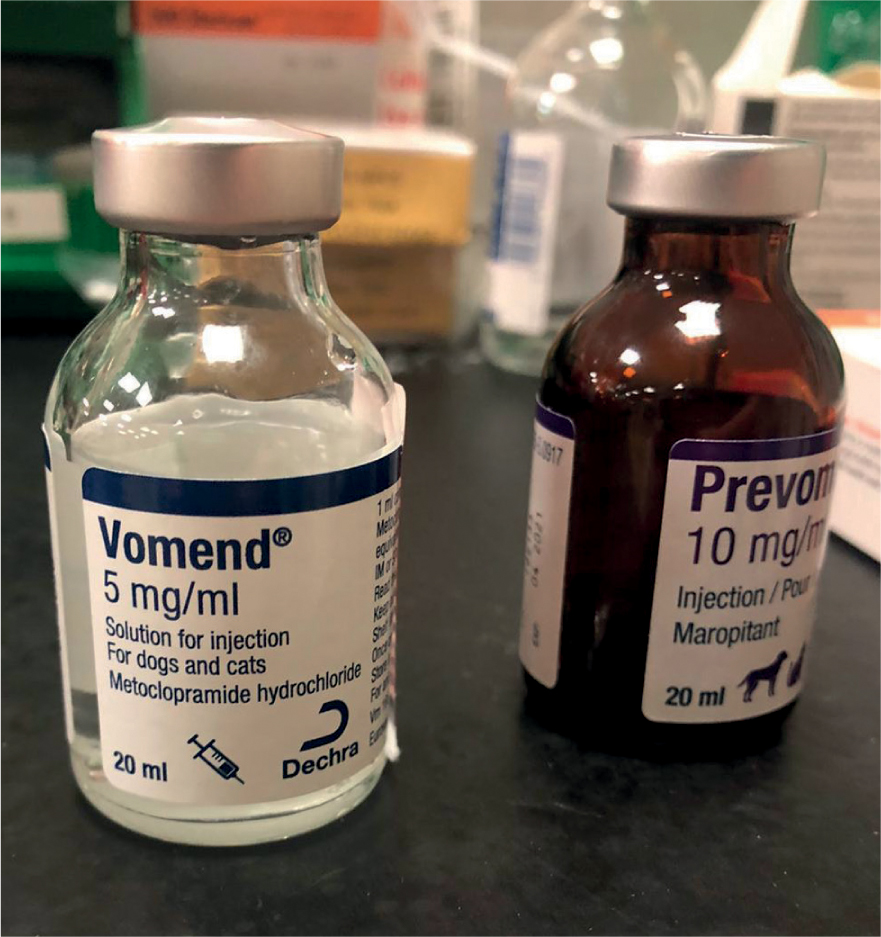
It was previously suggested that antibiotics should be used in the treatment of bacterial translocation, although this condition is hard to accurately identify, and there are now concerns over the use of antibiotics leading to antibiotic resistance. It is now suggested that they should only be used in cases of necrotising pancreatitis or those cases with a known infectious cause, not as a prophylactic treatment (Mansfield and Beths, 2015; Murphy, 2018).
Surgical intervention may be considered in cases of necrotising pancreatitis or where medical management is failing, however a small human study carried out recently showed no improved outcome for patients treated surgically rather than medically for cases of necrotising pancreatitis (Murphy, 2018).
Gordon (2011) suggested that it is important to look for underlying diseases and predisposing conditions in patients presenting with AP since these may require treatment to enable resolution of the AP.
Nursing care — analgesia and nutrition
Although there are many aspects to the nursing care of AP patients, this article will focus on analgesia and nutrition as two key areas where registered veterinary nurses (RVNs) can improve both patient welfare and outcome.
Pancreatitis is associated with significant pain both locally due to the inflamed pancreas and from visceral pain as a result of systemic inflammation (Mushtaq, 2017). Mansfield and Beths (2015) suggested that it is sensible to assume that all patients with AP will be experiencing some pain and because of the potential severity of this, a multi-modal analgesic approach should be taken. Due to the likely presence of hypovolaemia and dehydration in many patients presenting with AP, non-steroidal anti-inflammatory drugs should be avoided (Mansfield and Beths, 2015).
Full and partial µ agonist opioids are appropriate in the treatment of AP, and although respiratory depression is a known side effect of these drugs, Mansfield and Beths (2015) stated that this is rarely seen when used at the recommended doses and avoided in patients with respiratory disease or those receiving other central nervous system depressants.
Lidocaine may be used as a continuous rate infusion (CRI) in patients with AP as it is considered to have anti-in-flammatory properties as well as analgesic properties that act both peripherally and centrally (Mansfield and Beths 2015).
N-methyl-D-aspartate (NMDA) receptors have been reported to contribute to central sensitisation during visceral inflammation in rodent models of AP, leading to the use of ketamine, a potent NMDA receptor antagonist, in the treatment of AP. Ketamine can be administered as a CRI alongside other analgesic drugs (Mansfield and Beths, 2015).
Mansfield and Beths (2015) highlighted that both tramadol and gabapentin can also be used in the management of AP patients.
Thoracic epidural analgesia (TEA) has been used successfully in humans with AP, and found to provide excellent pain relief. The technique involves epidural catheter placement between T6 and L2 and has been found to reduce metabolic acidosis, reduce mortality and improve pancreatic and ileal perfusion in animal studies (Windisch et al, 2016). There are however safety considerations associated with placement of an epidural catheter and not all patients will be suitable candidates (Figure 6).
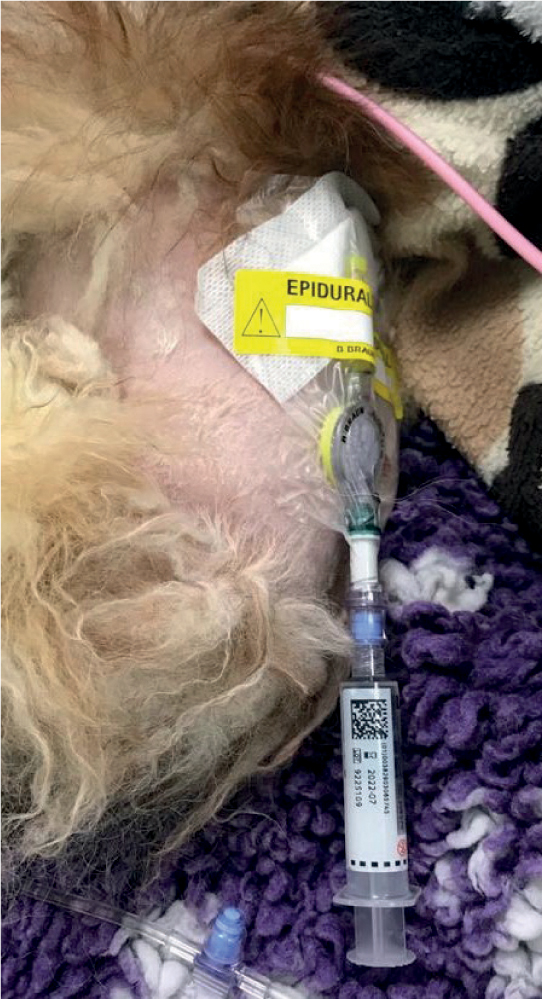
The role of the RVN in pain management stems around both monitoring, ideally with the use of a validated pain scoring system, and advocating for the patient. RVNs are often the eyes of the veterinary surgeon (VS) and should be able to inform them if the analgesia administered to these patients has achieved the desired effect. The use of a pain scoring system in practice allows for a controlled measure of pain, easily repeatable by different users. The Glasgow Composite Pain Scale, a behaviour-based acute pain scoring system, is most commonly used in veterinary practice. A short form of the scoring system was developed for use in practice to enable speed and ease of use (Balakrishnan and Benasutti, 2020).
Another key aspect, recognised in human medicine in the treatment of AP, is the provision of early enteral nutrition (EN). Wereszczynska-Siemiatkowska et al (2013) discussed the importance of nutrition in the treatment of severe AP since it is a hypercatabolic state with a negative nitrogen balance. It was previously suggested that patients with AP should be fasted to allow the pancreas time to ‘rest’. This theory has been challenged since rodent models have demonstrated that exocrine pancreatic secretion is actually reduced during pancreatitis (Mansfield and Beths, 2015). It is also now recognised that the gastrointestinal tract contributes significantly to the development of systemic inflammation that may occur secondary in AP, especially when there is a lack of nutrition supplied. Canine models of AP were found to have reduced bacterial translocation, reduced villus atrophy and reduced pancreatic inflammation when nutrition was delivered directly into the intestine (Mansfield and Beths, 2015).
Previously, parenteral nutrition (PN) was considered a useful treatment in humans with AP during the initial stages of pancreatic rest. Ong and Fock (2012) discussed studies that found all forms of EN would stimulate the pancreas to a degree, and this was only avoided by the use of PN. While PN remains an important nutritional option in cases of refractory vomiting, there are some serious complications associated with its use: hyperglycaemia, sepsis, bacterial translocation and intestinal atrophy (Jensen and Chan 2014; Ong and Fock 2012). Human studies have shown that patients suffering with AP receiving EN are less likely to suffer infectious complications and less likely to require surgical intervention, than when receiving PN. There is also a higher mortality rate associated with the use of PN in AP patients (Ramanathan and Aadam, 2019) (Figure 7).
There has been debate over the appropriate time to introduce nutrition in AP patients, but both Gordon (2011) and Mansfield and Beths (2015) suggested that EN should be introduced as early as possible. Jensen and Chan (2014) referred to guidance in human medicine, which suggested that EN should be started as soon as possible and ideally within the first 48 hours of hospitalisation.
Traditionally in human medicine, EN was administered via a jejunostomy tube, since it was demonstrated that nutrition administered in this way avoided exocrine pancreatic secretion (Mansfield and Beths, 2015). Mansfield and Beths (2015) discussed more recent studies that have demonstrated that prepyloric feeding is well tolerated in AP patients. Nasogastric (NG) and naso-oesophageal (NO) tubes are often well tolerated and easily placed with local anaesthetic, avoiding the need for a general anaesthetic. Limitations include a small lumen size restricting the choice of diet that can be administered. Oesophagostomy, gastrostomy and jejunostomy tubes can be considered in patients that are already undergoing general anaesthesia and surgical intervention (Jensen and Chan, 2014) (Figure 8).
Mansfield and Beths (2015) stated that studies have not demonstrated any increase in pancreatic secretion as a result of increased dietary fat intake, although they suggested that it makes sense to avoid a high fat diet since many AP patients are found to be hyperlipidaemic. Realistically, the diets used in practice are more likely to be considered based on their consistency and the lumen size of the feeding tube available.
Conclusion
AP is a challenging condition to manage, potentially multi-factorial in origin, and patients often present extremely painful. Early intervention of treatments is essential in cases of severe AP and RVNs can play a vital role in the management of these cases by ensuring the patient's nutritional needs are met and that appropriate analgesia is administered as prescribed by the VS.
KEY POINTS
- Canine acute pancreatitis (AP) is commonly seen in practice and can be a challenging condition to manage with patients requiring intensive nursing care.
- The pathophysiology of pancreatitis is complex but involves the early activation of pancreatic digestive enzymes with the pancreatic acinar cells.
- The two most commonly seen signs of AP in canine patients are vomiting and abdominal pain.
- A definitive diagnosis of either acute or chronic pancreatitis can be achieved only with histological examination of pancreatic tissue.
- Treatment for AP is mainly supportive, and analgesia administration should be considered as the condition is associated with significant pain.
- It is suggested that enteral nutrition should be introduced as early as possible in the management of canine patients with AP, with pre-pyloric feeding reported to be well tolerated.