Most veterinary patients experiencing acute pain may be managed with opioids, nonsteroidal anti-inflammatories (NSAIDs) and loco-regional anaesthetic techniques. These so called ‘traditional analgesics’ share one similarity, which is the management of pain being their primary indication (Lamont, 2008).
Adjunctive analgesics (also called analgesic adjuvants), are drugs that come from different pharmacological classes and have primary indications other than pain (Shaffran, 2005; Lamont and Mathews, 2007). They have the potential to improve comfort without adverse effects such as sedation, dysphoria, gastrointestinal tract dysfunction, respiratory depression, or renal toxicity associated with traditional analgesics (Wagner et al, 2002; Lamont, 2008; Wagner et al, 2010). They may also facilitate a reduction in the dose of other, concurrently administered, analgesics which may also reduce the incidence of adverse effects.
These agents are commonly, but not exclusively, co-administered with traditional analgesics in order to provide ‘multimodal analgesia’, where a range of analgesic and adjunctive drugs are used to target different points of the pain pathway and act at different levels involved in the modulation of pain perception (Acosta et al, 2005; Lamont and Mathews, 2007; Lamont, 2008; Steagall and Monteiro-Steagall, 2013) (Figure 1).
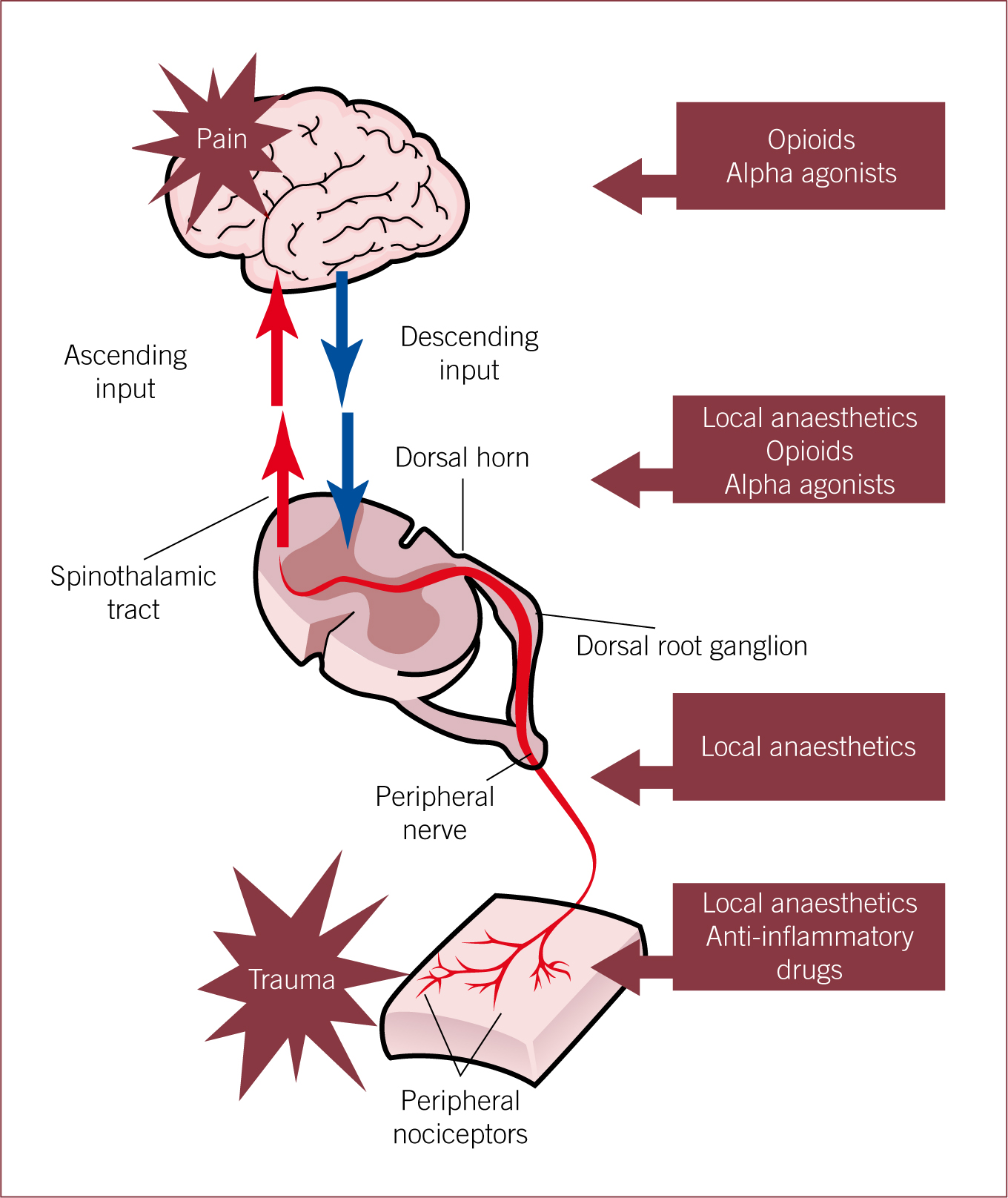
In simple terms, acute pain follows an acute injury, disappears with healing and tends to be self limiting (Gaynor, 2008). Chronic pain tends to last several weeks to months and persists beyond the expected healing time when non-malignant in origin (Gaynor 2008). Adjunctive analgesics have been used mostly in the management of chronic pain. However, their use in acute pain has been increasing and certain adjunctive agents are now used relatively commonly as analgesic supplements during the perioperative period (Lamont and Mathews, 2007; Lamont, 2008).
In humans, the World Health Organization (WHO) defined the levels of pain in cancer patients to be mild, moderate and severe. At each pain level a particular analgesic treatment is implemented (Zech et al, 1995). For patients experiencing mild pain, patients are treated with non-opioids (NSAIDs or paracetamol); moderate pain treatment would include ‘weak’ opioids added to NSAIDs or paracetamol and in severe pain, patients would be treated with ‘strong’ opioids, NSAIDs or paracetamol and adjuvants (Zech et al, 1995).
There are numerous adjunctive analgesics that may be considered in veterinary medicine and some, such as ketamine and α2-agonists, are familiar to practitioners whereas other have not been commonly used in veterinary medicine but have recently been used via the prescribing cascade (Lamont and Mathews, 2007; Lamont, 2008).
The definitions of terminology commonly used when describing pain have already been outlined in a previous article (Flaherty, 2013). This article will review the current application of adjunctive analgesics in veterinary medicine.
Pharmacology of adjunctive analgesics
Local anaesthetics (e.g. lidocaine, bupivacaine, mepivacaine, proxymetacaine)
Local anaesthetics are classified as sodium channel blockers and when administered perineurally (around a nerve or nervous tissues), they produce nerve conduction blockade by suppression of the permeability of excitable membranes to sodium (Nolan, 2000; Lamont, 2008). Note that local anaesthetics produce ‘anaesthesia’ and not ‘analgesia’ by the nature of their mode of action, although they are often noted as being part of a multimodal analgesic technique. A short description of some of the routes by which local anaesthetics can be administered is warranted, as this has not been discussed elsewhere. However, as the focus of this article is adjunctive analgesia, the systemic administration of lidocaine will be described in more detail.
Topical anaesthesia
- Cats are prone to laryngospam during endotracheal intubation so desensitisation of the larynx with lidocaine spray is common.
- Corneal desensitisation with proxymetacaine.
- Topical eutectic mixture of local anaesthetic (EMLA cream) — ratio of 1:1 oil-in-water emulsion of lidocaine and prilocaine. EMLA cream can be used to penetrate intact skin and after 45–60 minutes of contact time, the skin is anaesthetised enough for venepuncture.
Injection
- Local infiltration — around peripheral nerve endings in tissues providing local peripheral anaesthesia.
- Peripheral nerve blocks are capable of desensitising large areas. For example, a paravertebral nerve block would desensitise the paralumbar fossa of a cow prior to standing laparotomy.
Epidural and the subarachnoid
- Produces anaesthesia as it desensitises the spinal cord and is useful for procedures involving the caudal abdomen, pelvic limbs and perineum.
- Injection of local anaesthetic solution into the epidural space generally at the lumbosacral space (in dogs, cats, rabbits and pigs) or the sacrococcygeal, first or second intercoccygeal spaces (in horses, cattle) produces epidural (or extradural) anaesthesia.
- The extent of anaesthetic action depends on the spread of the drug and diffusion to neural tissues from the site of injection.
- Long-term administration of drugs is facilitated by placement of an epidural catheter.
- Opioids (such as preservative free morphine) can be added to provide analgesia in addition to anaesthesia.
- The subarachnoid space may be punctured instead when performing an epidural and drugs can still be administered via this route provided that doses are reduced accordingly.
Intra-articular
- Local anaesthetics are injected into a synovial joint. This technique is also used as a diagnostic tool during lameness investigation in the horse.
Systemically
The intravenous administration of lidocaine can be used for supplemental analgesia (note the change in terminology here). Note — Bupivacaine must never be administered intravenously (IV) due to its cardiotoxic effects.
- In dogs, intravenous infusion of lidocaine may produce analgesia. This can provide benefits during general anaesthesia and may be part of a multimodal approach to pain post operatively. It is often used during and following surgery of, or in animals with disease of, the gastrointestinal tract.
- Lidocaine can be mixed with other drugs for IV infusion (e.g. MLK — morphine, lidocaine, ketamine).
- In equine patients, intravenous infusion of lidocaine may increase intestinal motility and be useful in treating some forms of hypomotility, especially that associated with colic. The mechanism of action, although unknown, is thought to involve anti-inflammatory, analgesic or alteration to sympathetic inhibitory reflexes by suppressing the nerve transmission in afferent sensory pathways (Koenig and Cote, 2006).
As an adjunctive analgesic, lidocaine infusion has gained popularity in dogs as it is thought to inhibit modulatory nociceptive processing (the physiological process that leads to perception of pain after a mechanical, chemical or thermal stimulation), providing analgesia for neuropathic pain (e.g. intervertebral disc disease (IVDD)) as well as minimum alveolar concentration (MAC) sparing effects during general anaesthesia (Shaffran, 2005; Kerr, 2007). Lidocaine is reported to have cytoprotective effects (Cassutto and Gfeller, 2003), which may help prevent reperfusion injury, and reduce neutrophil chemotaxis and platelet aggregation, which could help significantly in cases with the potential for disseminated intravascular coagulopathy (DIC) or systemic inflammatory response syndrome (SIRS), including gastric dilation volvulus (GDV) and splenectomies. Lidocaine also has activity in preventing ileus (gastrointestinal hypomotility) potentially useful for enterotomies.
In dogs, an initial intravenous bolus dose of 1 mg/kg of lidocaine is given to rapidly achieve therapeutic levels, followed by doses as low as 10 µg/kg/min, which can provide analgesia in some cases; doses of up to 50 µg/kg/min may be required (Shaffran, 2005).
Wound soaker catheters (WSC)
WSC are soft, flexible, hollow, thin tubes with holes arranged along the length. The catheter is sutured into the deepest part of a wound and the injection port is left protruding. Local anaesthetic can be administered by intermittent injection or continuous infusion. This technique is frequently used in small animal patients and provides an alternative means of providing local anaesthesia in post-operative patients (Abelson et al, 2009). The use of WSCs has been reported in the horse (Zaccuro et al, 2007; Minghella and Auckburally, 2014).
The choice of local anaesthetic for a particular procedure is based on its pharmacokinetics. The absorption and speed of onset of action depends on its solubility and pKa (Nolan, 2000; Riedesel, 2008). Lipid solubility is directly proportional to the potency and duration of action of the local anaesthetic. The higher the lipid solubility, the more potent and the longer the duration of action of a particular agent (e.g. bupivacaine is more lipid soluble compared with lidocaine, therefore, it is more potent and has a longer duration of action). The pKa correlates with the speed of onset of action. In simple terms the pKa is the pH at which a drug exists in its unionised and ionised forms in equal parts. Local anaesthetics which exist more in their unionised form at physiological pH (7.4) will have a faster onset of action as a drug must be unionised to enter a cell.
Local anaesthetics can cause adverse effects if plasma concentrations reach a certain threshold value (Riedesel, 2008). The following are possible adverse effects seen with local anaesthetics:
- Central nervous system (CNS) — first signs of toxicity are skeletal muscle twitches which may be followed by tonic-clonic seizures. Note that this effect will be missed if toxic plasma levels are reached in patients under general anaesthesia.
- Cardiovascular system — bradycardia, hypotension and negative inotropic (reduced myocardial contractility) effects may be seen. Bupivacaine is more cardiotoxic than lidocaine and intravenous administration of bupivacaine is contra-indicated for this reason.
- Methaemoglobinaemia (a dyshaemoglobinaemia) may occur in cats and rabbits following the use of prilocaine.
- Cats have a lower plasma concentration toxic threshold which may cause seizures, severe bradycardia and studies caution against the use of intravenous local anaesthetics (i.e. lidocaine) infusion in feline patients (Pypendop and Ilkiw, 2005; Shaffran, 2005).
N-methyl-D-aspartate (NMDA) receptor antagonists (e.g. ketamine, amantadine, methadone)
One of the major receptor types involved in neuroplasticity (changes in neural pathways) and central sensitisation is the NMDA receptor. It is involved in the onset and maintenance of pathologic states of pain after the occurrence of lesions in peripheral nerves, and in the development of hyperalgesia and allodynia (Acosta et al, 2005; Flaherty, 2013). Inhibition of NMDA receptors in the spinal cord induces anti nociception in several types of persistent pain (Acosta et al, 2005). There are a number of drugs with potential NMDA antagonistic effects.
Ketamine
Classically, ketamine is used as a dissociative anaesthetic agent, but due to its antagonism of the NMDA receptor, it is also used widely in the treatment of pain at sub-anaesthetic doses. In humans, ketamine is effective in reversing some chronic pain states such as phantom limb pain and in burn patients (Nolan, 2000; Acosta et al, 2005; Kerr, 2007; Lamond and Mathews, 2007). There is some evidence that subanaesthetic doses of ketamine (lower than 1/10th of the anaesthetic induction dose) and/or constant rate infusion provides analgesia in dogs, cats and horses (Nolan, 2000; Dugdale, 2010). Peri-operative administration of ketamine is thought to prevent wind up from occurring, thus reducing post-operative pain. In humans, sub-anaesthetic doses of ketamine given by constant rate infusion peri- and intra-operatively have improved post-operative analgesia and reduced the need for opioids in patients undergoing abdominal surgery and other types of surgery. The analgesic effects appear to persist well beyond the expected duration of ketamine (Fu et al, 1997; Wagner et al, 2002).
The use of low-dose ketamine infusion for adjunctive analgesia has become common practice in veterinary medicine but there are limited studies evaluating the analgesic effects of ketamine in the peri-operative period (Lamond, 2008). Wagner et al (2002) demonstrated that dogs undergoing forelimb amputation which had received peri-operative ketamine were more comfortable during the post-operative period. Pain scores were significantly lower 12 and 18 hours post operatively. Slingby and Waterman-Pearson (2000) reported that a single dose of ketamine before anaesthetic induction or after extubation in dogs undergoing routine ovariohysterectomy reduced pain scores, rescue analgesia requirement and post-operative wound hyperalgesia compared with control dogs. Significant dose-dependent isoflurane MAC-sparing effects have been reported when ketamine infusions are administered during general anaesthesia in dogs (Lamont, 2008).
Ketamine has been widely used in cats as a dissociative anaesthetic agent but its analgesic effects in this species have been poorly investigated (Robertson and Taylor, 2004; Robertson, 2008). It has been reported that when ketamine was incorporated as part of the anaesthetic protocol it provided better post-operative analgesia when compared to thiopentone and halothane with or without butorphanol. This suggests that using ketamine as part of the anaesthetic protocol may provide pre-emptive analgesia in the cat (Robertson and Taylor, 2004).
The administration of low doses of ketamine to horses with colic pain has been described, particularly when the pain is refractory to other, more traditional analgesics. This is sometimes termed as a ‘ketamine stun’. Ketamine must never be administered alone to horses but should be layered over alpha-2 agonist sedation/analgesia (Abrahamsen, 2007). Care should be taken when using ketamine to treat colic pain in horses. Repetitive doses can accumulate and lead to recumbency, especially if doses are given close together. Low doses (0.2 mg/kg IV) of ketamine can be repeated every 15 to 20 minutes providing alpha-2 agonist sedation is still present. As xylazine is short acting (approximately 20 minutes if given IV), repeated small doses should be used when using the ketamine stun technique.
Coetzee et al (2010) showed that the combination of sub-anaesthetic doses of ketamine (0.1 mg/kg IV) in combination with xylazine (0.05 mg/kg IV) provided adequate analgesia when administered prior to castration of calves.
In addition to systemic administration, ketamine may be administered by several other routes to supplement analgesia such as epidural, intra-articular, topical and infiltrative injection. Clinical trials have been conducted for oral, intranasal, transdermal, rectal and subcutaneous routes (Kronenberg, 2002). Hamilton et al (2005) evaluated the analgesic effects of epidural ketamine in dogs and the results are suggestive of a potential but limited role for ketamine in epidural protocols. The current licensed preparations of ketamine are unsuitable for epidural use due to the presence of preservatives within the solution.
Amantadine
Amantadine was developed as an antiviral agent for the treatment of influenza A virus in humans. Since its development amantadine has been licensed in Europe for the treatment of Parkinson's disease and neuropathic pain, although its use in humans for the treatment of pain is diminishing (Lamont and Matthews, 2007; Grubb 2010a; Holopherne-Doran 2010). The analgesic effects of amantadine are mediated via NMDA receptor antagonism. In humans, it is well absorbed and distributed after oral administration with primary elimination via the kidneys. However, there are no current pharmacokinetic data available for dogs and cats (Lamont and Matthews, 2007; Grubb, 2010a). Grubb (2010b) reported the use of amantadine in dogs with chronic osteoarthritis and, when administered in conjunction with a NSAID, the level of pain was reduced when compared with the use of NSAID alone. The reported dose of amantadine used was 3–5 mg/kg orally once a day for a minimum of 21 days (Grubb 2010b). The recommended dose in cats is 3–5 mg/kg orally daily (Jordan and Ray, 2012).
Side effects are rarely reported in dogs and cats although this may reflect its minimal use. In humans occasional loose stools, flatulence and agitation are reported. Side effects may be more likely to occur in animals with renal dysfunction as drug accumulation may occur (Lascelles et al, 2008; Grubb, 2010a). Amantadine has a low safety margin so accurate dosing is essential in order to prevent toxicity (Jordan and Ray, 2012). The toxic dose in cats is reported as 30 mg/kg; at 15 mg/kg behavioural effects have been noted (Jordan and Ray, 2012). Amantadine may interact with anticholinergics, potentiating this activity resulting in urine retention, increased intraocular pressure, constipation and excitement in cats (Jordan and Ray, 2012).
Further studies are required in order to assess the full potential of amantadine as an adjunctive analgesic in the control of chronic pain in veterinary patients.
Methadone
Methadone is a synthetic μ-agonist opioid which has similar pharmacological properties to morphine (Lamont and Matthews, 2007). Apart from its opioid receptor effects, methadone has been demonstrated to produce NMDA antagonistic effects and may be useful in the management of central sensitisation and chronic pain syndromes (Muir, 2010).
Tramadol
Tramadol is an atypical, centrally acting, synthetic opioid. It is a codeine analogue that has been used to treat pain in humans for over 20 years (Borer-Weir, 2014). Despite the misconception that its main analgesic action is opioid receptor mediated, its primary mechanism of action is inhibition of neuronal reuptake of noradrenaline and 5-hydroxytryptamine (serotonin). Tramadol also stimulates pre-synaptic serotonin release (Lamont and Mathews, 2007). Serotonin reuptake inhibition and or release provides an alternative pathway for analgesia. Tramadol has weak opioid receptor effects. Tramadol may be useful in the management of acute and chronic pain of moderately severe intensity associated with a variety of conditions, including osteoarthritis, fibromyalgia, diabetic neuropathy, neuropathic pain and even perioperative pain in human patients (Lamont and Mathews, 2007; Dugdale, 2012).
Tramadol is metabolised to O-desmethyltramadol, an active metabolite thought to be responsible for the main analgesic effects (Reidesel, 2008). The pharmacokinetics of tramadol have been well investigated in the dog, but conflicting data have been reported. Studies have been carried out in cats, horses and sheep (McMillan et al, 2008; Shilo et al, 2008; Habibian et al, 2011). Tramadol oral bioavailability in the dog is around 65% but there is significant individual variability. It is metabolised in the liver by demethylation and glucuronidation and excreted by the kidney (Plumb, 2008; Riedesel 2008). The elimination half life in dogs after oral or intravenous administration is much shorter than in humans. This suggests that in order to maintain therapeutic plasma concentrations, frequent administration of tramadol is necessary in dogs (McMillan et al, 2008; Borer-Weir, 2014). In cats, the clearance of tramadol appears to be slower than dogs which is likely due to the species difference in hepatic metabolism — glucuronidation is less efficient in cats (Pypendop and Ilkiw, 2005; Borer-Weir, 2014). In horses, the elimination half-life of tramadol was very short, suggesting that frequent administration is necessary to maintain effective plasma concentrations of tramadol. Its bioavailability is low and oral administration is not recommended in this species (Shilo et al, 2008).
The main side effect in the dog is mild sedation, but other central nervous system effects (agitation, anxiety, tremor, dizziness) and gastrointestinal effects (inappetance, vomiting, constipation and diarrhoea) have been reported (McMillan et al, 2008, Riedesel, 2008). In cats, Teppema et al (2003) reported that although cardiorespiratory side effects are minimal, there may be an increase in the apnoeic threshold and reduction of total carbon dioxide (CO2) sensitivity which is thought to be mediated via opioid receptors.
The recommended oral dose of tramadol for the dog varies widely, but current suggested dose rates are 2–4 mg/kg every 8–12 hours, with the higher dose providing correct therapeutic concentrations (Flaherty and Murrell, 2014). A similar dose range can be used in the cat although the bitter taste of tramadol makes oral dosing difficult. Suggested dose ranges of tramadol in horses are between 2 and 5 mg/kg IV and it may be a useful drug to treat laminitic pain refractory to other therapy (Guedes et al, 2012).
Tramadol has the potential to interact with other drugs that inhibit central serotonin or noradrenaline reuptake, leading to ‘Serotonin Syndrome’. It should be avoided in patients that may have received monoamineoxidase inhibitors (MAOIs) such as selegiline or opioids which inhibit serotonin reuptake (methadone, fentanyl and pethidine).
Gabapentin
Gabapentin (1-aminomethyl-cyclohexane acetic acid) is licensed in humans to treat epilepsy and neuropathic pain. Its analgesic effects are thought to prevent allodynia as well as hyperalgesia (Plumb, 2011). Gabapentin has a similar structure to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) but its mechanism of action is not mediated through the GABA receptor. Its actual mechanism of action is poorly understood although recent studies have shown that the analgesic effects of gabapentin appear to be mediated by different pathways (Kerr, 2007; Lamont and Mathews, 2007; Lamont, 2008; Peck et al, 2008; Grubb 2010a, Holopherne-Doran, 2010; Lorenz et al, 2012):
- Supra-spinal potentiation of the descending pain inhibitory pathway
- Spinal cord mechanisms — modulation of calcium current by binding to calcium channels or direct inhibition of NMDA receptors.
Few clinical studies have been conducted in veterinary patients with acute and chronic pain. Despite this, gabapentin use has increased widely in veterinary patients for its analgesic properties in chronic pain but more recently it has been used for perioperative analgesia in dogs and cats (Lamont, 2008), in cats for chronic pain due to musculoskeletal and neuropathic pain (Lorenz et al, 2012; Steagall and Monteiro-Steagall, 2013), and in horses with laminitis, neuropathic or chronic pain states (Terry et al, 2010). Recommended doses for adjunctive treatment of chronic pain in dogs is 3 mg/kg orally once daily but doses up to 10 mg/kg orally 2 to 3 times a day may provide better analgesia (Posner and Papich, 2009).
Amitriptyline
Amitriptyline is a tricyclic antidepressant (TCA) most commonly used for the treatment of behavioural conditions such as separation anxiety or generalised anxiety in dogs, excessive grooming and spraying in cats. It may also be useful for adjunctive treatment of pruritus or chronic pain of neuropathic origin in dogs and cats and idiopathic cystitis in cats (Chew, 1998; Plumb, 2011). TCAs are recommended as the first line of treatment for neuropathic pain in humans (Epstein, 2009; Plumb, 2011). The analgesic activity of amitriptyline is via blockade of noradrenaline and 5-hydroxytryptamine (serotonin) reuptake, although other mechanisms have been proposed, and therefore its use can also lead to the development of serotonin syndrome.
In cats, amitriptyline is rapidly absorbed from both the gastrointestinal tract and from parenteral injection sites, but transdermal (PLO-gel based) absorption is poor (Mealey et al, 2004; Plumb, 2011). After oral administration in cats, peak level concentration occurs within 1 to 2 hours compared with 2 to 12 hours in other species (Mealey et al, 2004). Amitriptyline is highly protein bound and enters the CNS and the maternal milk, and it is metabolised in the liver to several metabolites, including nortriptyline which is active (Mealey et al, 2004; Plumb, 2011). Half life in dogs has been reported to be 6 to 8 hours (Plumb, 2011).
Occasionally, side effects may be seen — sedation, constipation, urinary retention, hyperexcitability, hypersalivation, cardiac dysrhythmias, diarrhoea and vomiting. Bone marrow suppression leading to thrombocytopaenia may also be observed (Plumb, 2011). Its use is contraindicated if monoamine oxidase inhibitors are being administered, pre-existing seizures, thyroid disorders, hepatic disorders, keratoconjunctival seca, glaucoma, cardiac arrhythmias, diabetes or adrenal tumours (Plumb, 2011).
α2-agonists (medetomidine, dexmedetomidine)
α2-agonists are licensed primarily for use as a sedative in dogs and cats, but also have potent analgesic properties. The mechanism of action is mediated via α2-adrenergic receptors located in the dorsal horn of the spinal cord which modulate the release of neurotransmitters (Kerr, 2007; Plumb, 2011).
Medetomidine, and its dextrorotatory enantiomer dexmedetomine, are labelled for both sedative and analgesic use in dogs and cats, to facilitate clinical examinations and procedures, minor surgical procedures and minor dental procedures (Plumb, 2011). The pharmacologic effects of both medetomidine and dexmedetomidine are very similar and are listed below, although dexmedetomidine is twice as potent as medetomidine and shorter acting (Kerr, 2007; Plumb, 2011).
- CNS depression — leading to sedation and anxiolysis
- Analgesia
- Decreased gut motility and reduced secretions
- Decreased endogenous insulin release
- Peripheral vasoconstriction with bradycardia and a decrease in cardiac output
- Respiratory depression
- Diuresis
- Hypothermia
- Muscle relaxation.
In the cardiovascularly stable patient, Quandt (2009) suggested medetomidine at infusion rate of 1 to 3 μg/kg/hour IV following an initial loading dose of 1 μg/kg IV. Valtolina et al (2009) compared the analgesic effects of dexmedetomidine infusion with morphine infusion and it was suggested that dexmedetomidine is equally effective in providing post-operative analgesia in dogs and can contribute to a balanced post-operative analgesia regimen when used at a dose rate of 25 μg/m2/hour IV following an initial loading dose of 25 μg/m2 IV. However, because the α2 agonists also cause CNS depression, animals may be difficult to assess for pain if profound sedation occurs.
Conclusion
Adjunctive analgesia has the potential to relieve pain and improve patient comfort in veterinary patients, especially when traditional analgesics alone have failed. This article reviewed some of the current pharmacological agents used as adjunctive analgesics. As most are either not licensed for veterinary use at all, or are licensed for other indications, it is important to ensure justification and correct use of the prescribing cascade. Veterinary nurses should also be familiar with the relevant clinical pharmacology of each drug in order to communicate to owners the potential risks, side effects and reasoning for therapeutic use.
Key Points
- Adjunctive analgesics or analgesic adjuvants are drugs from different pharmacological classes and have primary indication other than pain.
- Adjunctive analgesics can improve comfort without side effects associated with traditional analgesics (i.e. opioids, NSAIDs).
- Adjunctive analgesics are usually combined with traditional analgesics to provide multimodal analgesia.
- The specific mechanism of action of many adjunctive analgesics remains unclear.
- Most adjunctive analgesics are either not licensed or are licensed for other indications in veterinary patients, it is important to ensure correct use of the prescribing cascade.

