References
Cystic ovarian disease in female guinea pigs
Abstract
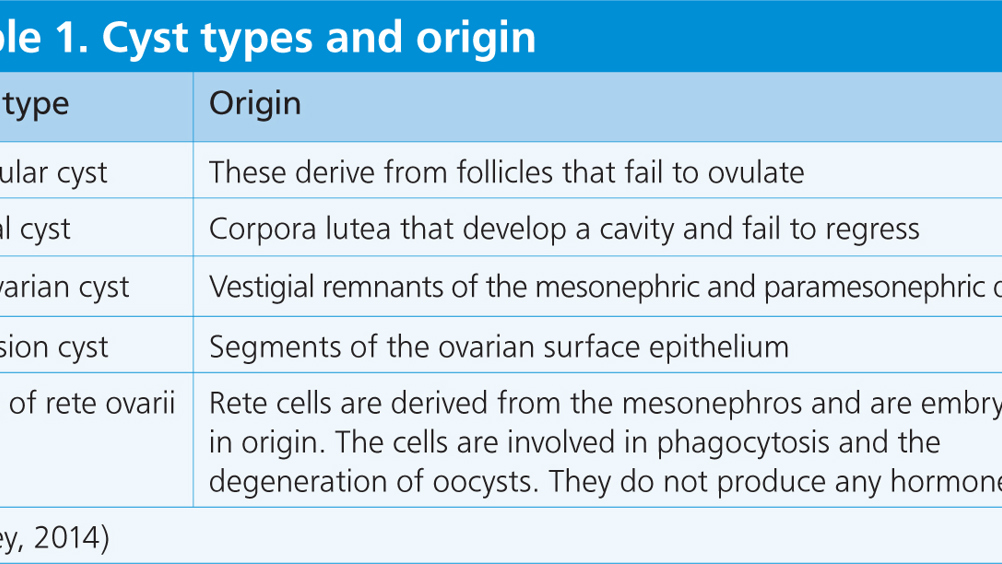
This article will look at cystic ovaries in female guinea pigs. Cystic ovaries can be functional or non-functional fluid filled cysts that usually develop spontaneously in the older sow. The presence of cysts usually reduces fertility and potentially causes serious uterine disease. Identifying common symptoms related to this condition can aid the veterinary nurse when performing clinical examinations. Species specific care is vital to securing optimum patient care and the chance of a good outcome.
Veterinary nurses (VNs) working in referral or first opinion practice carry out routine physical examinations of patients daily. This can be as simple as taking a temperature, checking mucous membrane colour or performing a full health check as part of a nursing consultation. As prey species, guinea pigs instinctively hide signs of pain/disease and injury, therefore at every opportunity a clinical examination is prudent. This, together with a sound knowledge of common diseases and conditions in this species will provide the cornerstone to gold standard nursing care and improvement of clinical satisfaction and client trust.
Cystic ovarian disease is very common in sows over the age of 1.5 years, an incidence of 76% has been reported at post mortem (Keller et al, 1987). Puberty occurs at around 2 months of age in females and peak reproduction is between 3 and 20 months, although some sows may continue to breed until 4–5 years of age. Guinea pigs are seasonally polyoestrous but breed year round in captivity. The sow possesses a single pair of inguinal mammary glands, paired ovaries, a bicornuate uterus consisting of paired uterine horns, a uterine body and a single cervix (Pollock, 2017).
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

