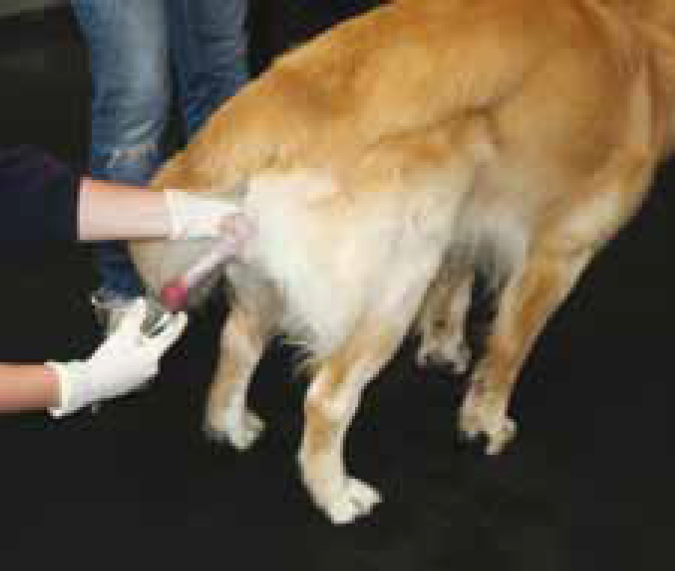
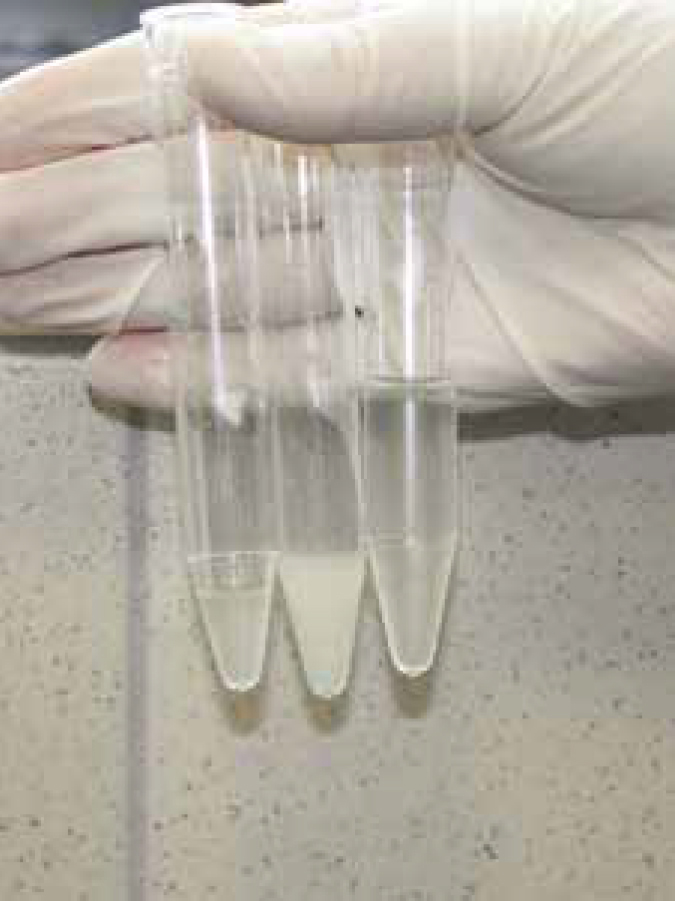
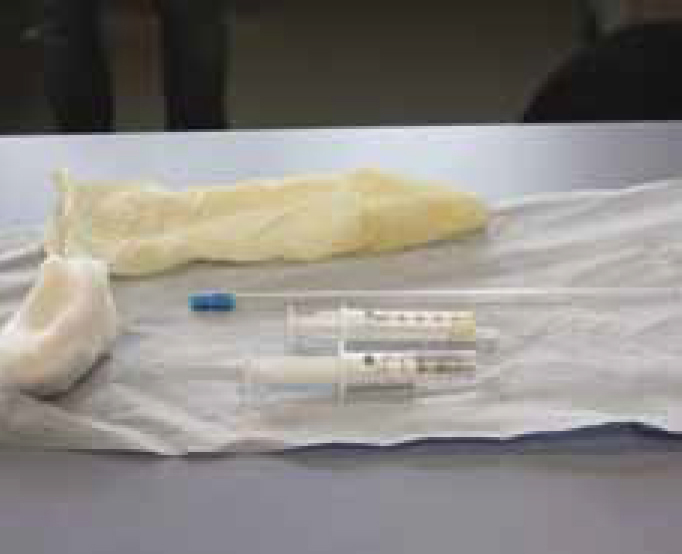
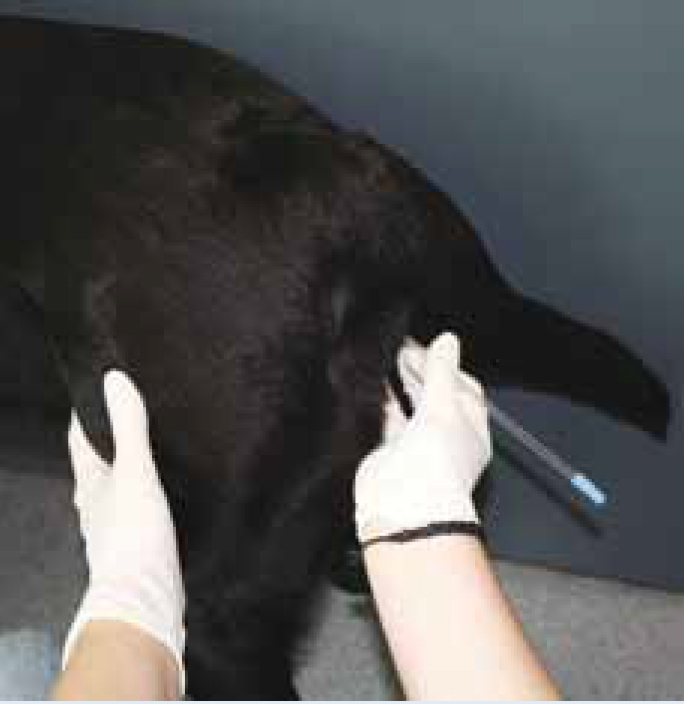
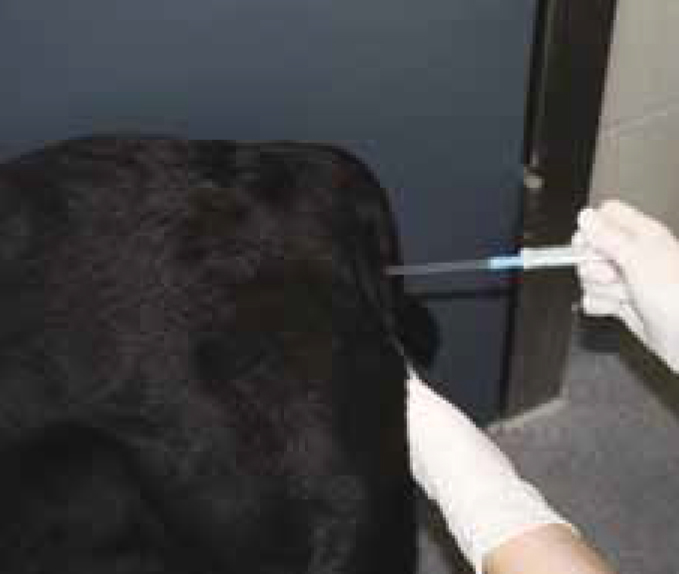
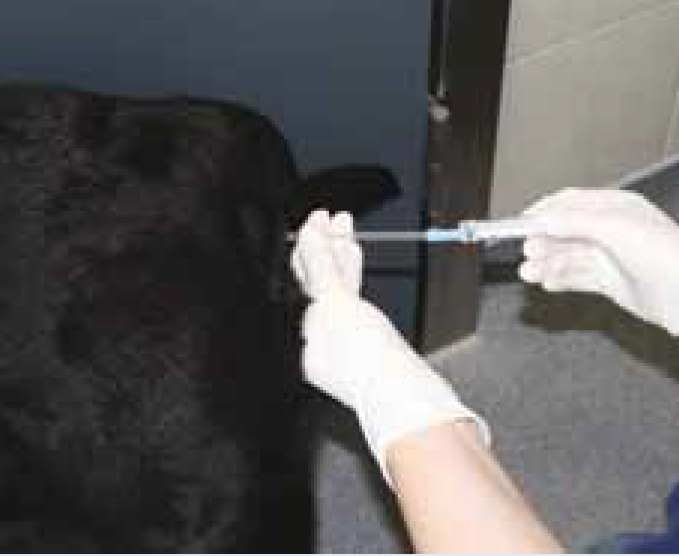
Artificial insemination (AI) is a method of introducing semen, previously collected from the male, and depositing it into the bitch's vagina or uterus to achieve a pregnancy.
There are many reasons that AI in the dog may be required (Figure 1). For example, it could be that a planned mating has not resulted in a ‘tie’, the male or female are inexperienced or the male, while still fertile, is not physically able to mate the bitch, or it could simply be that semen has been imported from another country to enable breeders to keep their gene pools diverse.

Occasionally, and particularly if the reason for insemination is due to the inexperience of the male, it may not prove straight forward to collect a good semen sample. Therefore, ideally, before insemination is considered, the male should be proven fertile either by means of a fertility test or from a previous successful mating, and the dog should have been collected from at least once so that the process of collection is familiar to him. Unfortunately, fertility testing is not usually offered as a service in private veterinary practice, therefore, if a fertility test is required it is likely this would need to be arranged with a reproductive veterinary specialist.
AI must always be performed by an experienced professional. Incorrect collection techniques could cause pain to the male and could prevent a collection being successful or future collections being successful. A painful experience could also lead to difficulties in future matings. Improper insemination technique could cause pain to the bitch or result in damage to her vaginal tract. Improper insemination techniques could also result in failure to conceive in that breeding season. In the UK, in order to register a litter born from AI, the Royal College of Veterinary Surgeons have advised that AI is a veterinary procedure and therefore should only be performed by a veterinary surgeon (Kennel Club, 2012).
Any breeder wishing to register a litter of puppies as the result of AI should first refer to the Kennel Club's regulations surrounding the use of AI. The Kennel Club will not register a litter resulting from AI unless certain criteria are met. For example, the General Committee will not normally accept an application to register a litter if the donor male is alive and domiciled in the UK, with one exception, namely that Irish Wolfhounds of 8.5 years or older and domiciled in the UK may be used as donors in AI (Kennel Club, 2012). Refer to the Kennel Club's website for further information (http://www.thekennelclub.org.uk/item/478).
Methods of AI
There are three main methods of AI: vaginal insemination; trans-cervical insemination [TCI] also commonly known as uterine insemination; and surgical insemination. In addition, and depending on the time delay between collection and insemination, the semen may need to be preserved. Therefore, there are also three semen types: fresh semen; chilled semen (preserved with an extender and kept chilled); and frozen semen (preserved with an extender and cryoprotect-ants and stored, frozen in liquid nitrogen) (Table 1). The extender is a liquid that contains buffers, sugars and cryoprotectants to nourish and protect the sperm while they are kept chilled or frozen.
| Semen type | Survival time (approximately) |
|---|---|
| Fresh semen | 12 hours at room temperature |
| Chilled semen | 4 days (kept chilled at 5oC) |
| Frozen semen | Theoretical indefinite life at a temperature of -196oC |
Due to the varying survival times of sperm within the semen types (Table 2), some methods of insemination will be unsuitable (Table 3).
| Semen type | Survival time In reproductive tract (approximately) |
|---|---|
| Fresh semen | 7 days |
| Chilled semen | 2 days |
| Frozen semen | (post thaw) 6 hours |
| Semen type | Type of insemination suitable |
|---|---|
| Fresh semen | Vaginal insemination |
| Chilled semen | Vaginal insemination |
| Frozen semen | Trans-cervical/ uterine or surgical insemination |
The timing of the insemination should also be carefully considered depending on the type of semen used and the method of insemination chosen. For example, due to the relatively short survival time of chilled and frozen semen, it is imperative that the bitch has already ovulated within the preceding 24–48 hours, thus ensuring the ova are available to be fertilized immediately. There are several methods available to enable the optimum mating time to be detected. One of these methods is blood hormone levels, the most common type of testing available is serum progesterone levels, most commonly the qualitative test. This test detects a colour change in the sample which gives the user a result of low progesterone, intermediate progesterone or high progesterone levels. These will give an indication of the level of progesterone in the blood and therefore detect ovu-lation — at high progesterone levels (usually 4–8 ng/ml) ovulation occurs (two matings are preferable, 48 hours apart). Vaginal cytology is another method available. By looking directly at the cells that line the vagina and the changes that happen, it is possible to detect the optimum mating time.
Semen
A dog ejaculates in three distinct fractions. The first fraction is clear fluid and originates from the prostate gland; its function is to flush any urine or cell debris from the male's urethra (Simpson et al, 1998).
The second fraction is the sperm rich fraction, usually cream in colour, containing millions of sperm and the fraction that is required for insemination (Simpson et al, 1998).
The third fraction is again clear fluid, originating from the prostate gland and its function is to wash the sperm through the bitch's cervix into her uterus and forwards into her uterine tubes where fertilization will take place (Simpson et al, 1998). However, while the second fraction is used for insemination, the third fraction is not used as a flushing fraction. This is replaced with sterile saline, to ensure there is no chance of any detriment to the sperm.
For this practical article, AI using fresh semen and the vaginal insemination method is discussed.
Things to remember
There are a number of things to remember when carrying out AI:
Equipment required
The following equipment are required for AI:
The procedure
See Step-by-Step guide for detail on carrying out AI.
Insemination should be repeated after 48 hours, providing the bitch is still in oestrus. This will maximize the coverage of the fertile period in the bitch and maximize the chances of conception (Simpson et al, 1998).
Conclusion
AI is used for a number of reasons, and it is essential that it is carried out by an experienced professional/practitioner in order to be successful and without problems. The correct timing is key to the success of AI, and survival times of semen and timing of ovula-tion should be carefully coordinated.