Non-steroidal anti inflammatory drugs (NSAIDs) are a class of drugs that are able to reduce inflammation without the need for steroids, by competitively inhibiting the formation of the inflammatory mediator prostaglandin.
Their use is appealing in veterinary medicine in acute and chronic inflammatory conditions that cause pain because of the therapeutic analgesic activity, their long duration of action, they come without sedative or behavioural modifying properties, and there are different formulations available (injectable, and oral tablet or liquid forms).
Because of the range of NSAIDs available in the veterinary market, careful drug and dose selection (and frequency) must be made, which will depend on the species it is being prescribed for. Although these are prescribed by the veterinary surgeon, it is useful for the veterinary nurse to be familiar with the NSAIDs available, and their pharmacology, to effectively nurse their patients and help answer any owner queries.
Specific NSAID drug doses, formulations for different species and the administration route are not discussed in this article and the reader is advised to consult a veterinary formulary.
Key words used throughout this article and their definitions are shown in Table 1.
Table 1. Key words
| Key word | Definition |
|---|---|
| Nociceptor | A sensory neuron that detects potentially painful or damaging stimuli |
| Hyperalgesia | An exaggerated response to a normally painful stimulus |
| Allodynia | The sensation of pain from a non-painful stimulus |
| Pharmacodynamics | The effect of a drug in the body |
The inflammatory process
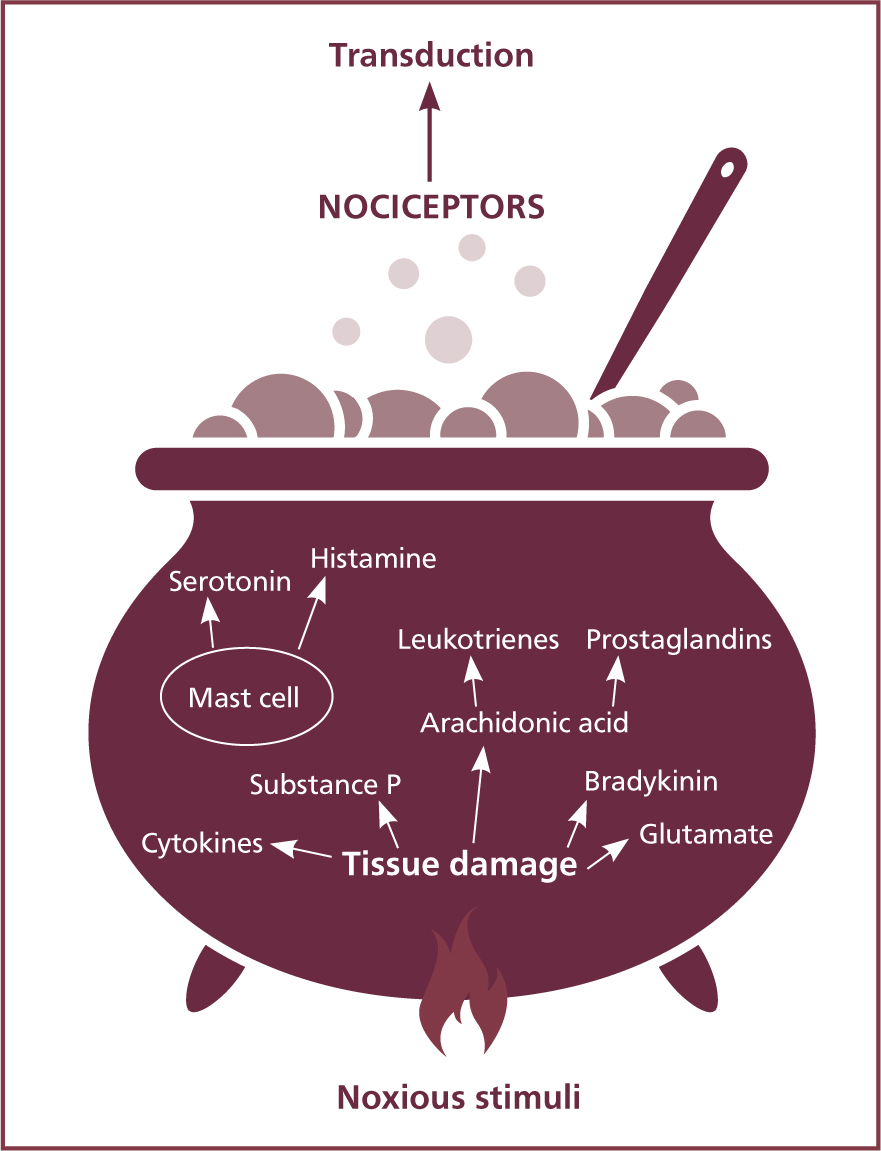
When the phospholipid (PLA2) layer of the cell membrane of tissue is injured or damaged, it leads to the activation and release of inflammatory mediators. These create an acidic environment referred to as an inflammatory soup, which hypersensitise nociceptors, causing hyperalgesia. The ‘ingredients’ of the soup are bradykinin, serotonin, histamine, cytokines, arachidonic acid, substance P and glutamate as shown in Figure 1.
The inflammatory mediator of importance when discussing NSAIDs is arachidonic acid: the precursor to the production of leukotrienes and prostanoids. There are two enzymes that determine which inflammatory mediator the arachidonic acid produces as seen in Figure 2.
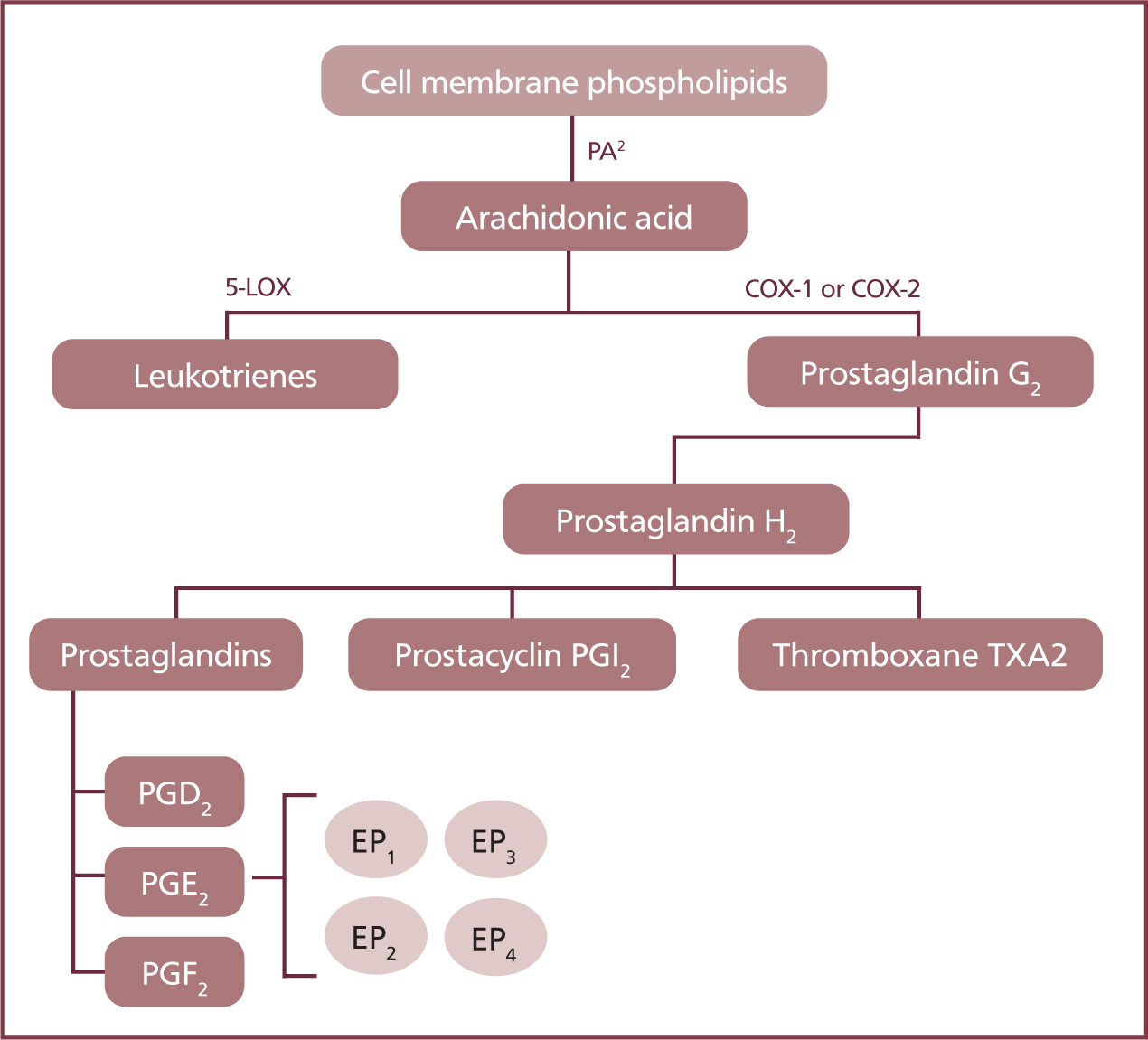
- The enzyme 5-lipoxygenase (5-LOX) transforms arachidonic acid into leukotrienes
- The cyclo-oxygenase (COX) enzyme can form three different prostanoids — prostaglandins, prostacyclins, thromboxanes.
The cyclo-oxygenase enzyme
It is the COX enzyme that converts arachidonic acid into different prostanoids, which regulate several homeostatic processes in the body.
There are two isoforms of the COX enzyme, COX-1 and COX-2:
- COX-1 is found everywhere in the body and assists in many biological functions. Most noticeably, it forms prostacyclins (PGI2, and potentially PGE2 too) that are responsible for the regulation of renal blood flow, mucosal blood flow, mucus production and cell turnover in the gastrointestinal (GI) tract, and produce thromboxanes (TXA2), which assist in platelet aggregation. COX-1 is sometimes referred to as the ‘constitutive good housekeeping’ enzyme.
- COX-2 produces proinflammatory prostaglandins (PGE2, PGF2, PGD2) to intensify the pain response, and when locally released into the tissue they increase blood flow to the area allowing neutrophils and other immune compounds to penetrate the tissue. They are also known as the ‘inducible bad’ enzyme.
However, there is some overlap in the roles of COX-1 and COX-2 in the body. For example, thromboxanes are dependent on COX-1 for platelet aggregation but they are also involved in the initial role of inflammation, hyperalgesia and allodynia, and COX-2 is also produced in the central nervous system (CNS), kidneys, the reproductive tract and may also repair gastrointestinal erosions (Kerr, 2016).
Despite this, COX-2 is still the primary enzyme involved in the inflammatory response. This means unfortunately that newer NSAIDs that selectively target the COX-2 pathway to reduce pain and inflammation may still have adverse effects on other parts of the body.
Pharmacology of NSAIDs
NSAIDs are highly protein bound to albumin in plasma (95–99%) (Lees, 2009), with varying half-lives dependent on the drug and species it is used in. At the site of inflammation, plasma proteins leak into the tissue (albumin is a part of exudate fluid) taking the bound NSAID with it and ‘trapping’ it there (Dugdale, 2010). This results in an anti-inflammatory duration that is longer in tissue than what is measured in plasma (Kerr, 2016).
NSAIDs typically undergo hepatic metabolism and metabolites are renally excreted. The peak plasma concentrations, metabolism, and excretion of NSAIDs may be prolonged in animals with hepatic or renal disease.
While NSAIDs have very similar clinical effects, they can be differentiated and classified into three groups based on their chemical properties:
- Carboxylic acids (propionic acids; carprofen)
- Enolic acids (oxicams; meloxicam)
- Cyclo-oxygenase-2 enzyme inhibitors (pyrazoles; firocoxib, robenacoxib).
COX inhibiting drugs are classified as non-selective, or selective/preferential to either the COX-1 or COX-2 isoform, as shown in Table 2.
Table 2. Commonly used human and veterinary NSAIDs and their classification
| Classification | Inhibition | Drug example |
|---|---|---|
| Non-selective COX inhibitor | COX-1 and COX-2 | Aspirin, ibuprofen |
| Selective COX-1 inhibitors | Inhibits COX-1 more than COX-2 | None in the veterinary market |
| Selective COX-2 inhibitors | Inhibits COX-2 more than COX-1 | Carprofen, meloxicam |
| Highly selective COX-2 inhibitors — ‘The Coxibs’ | Inhibits COX-2 with minimal COX-1 effect | Robenacoxib, firocoxib, mavacoxib |
Desirable pharmacological effects
Different NSAIDs vary in their chemical properties, however, they have similar peripheral and central pharmacodynamics:
- Peripheral actions include anti-inflammatory effects, analgesia, antithrombotic properties
- Central actions include antihyperalgesic and antipyretic effects.
Anti-inflammatory
The anti-inflammatory effect provided is through the inhibition in the synthesis of prostaglandins, preventing vasodilation, histamine and bradykinin production. The histamine released from mast cells promotes the redness, swelling and oedema seen with tissue injury, and the bradykinins attach to nociceptors and potentiate impulses to the CNS. NSAIDs also inhibit the activation of the nuclear factor kappa B protein (NF-κB) which also produces inflammatory mediators and immune responses (Dugdale, 2010).
Analgesia
The nociceptive pathway is responsible for the communication of a noxious stimuli from the site of injury to the brain. Nociceptors found in the skin, muscle, joints, and tissue detect mechanical (pressure onto the tissue), chemical (endogenous inflammatory mediators or external chemicals) or thermal (extremes of hot or cold) stimuli.
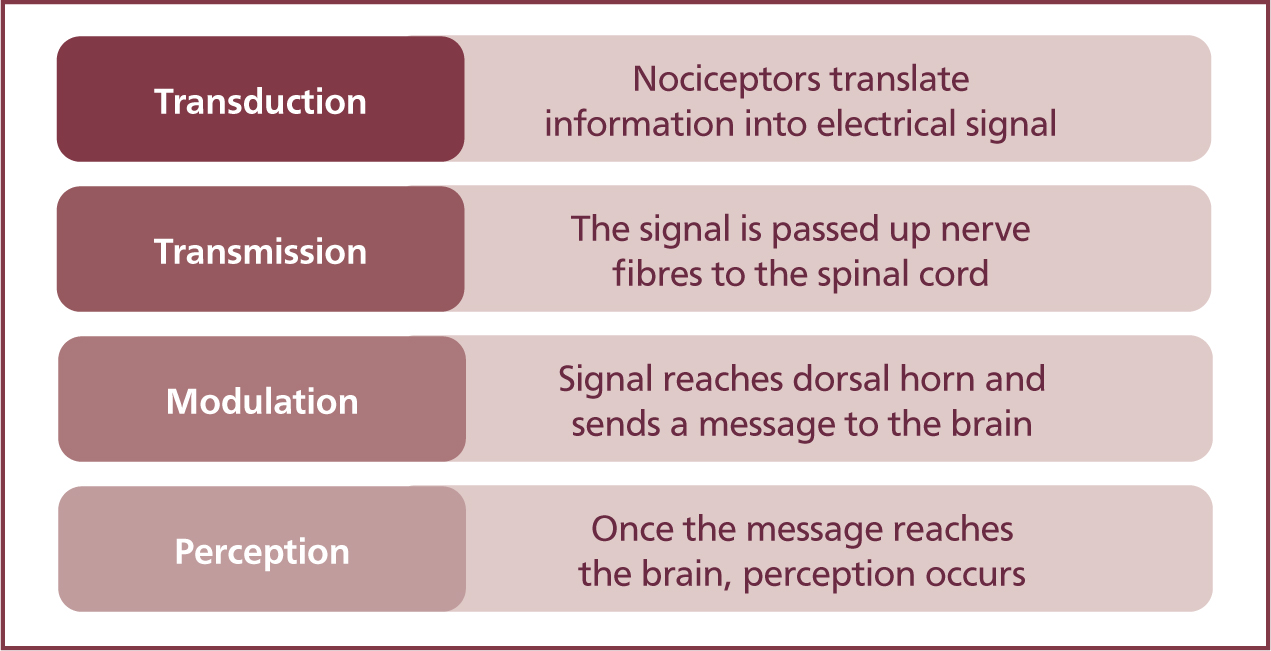
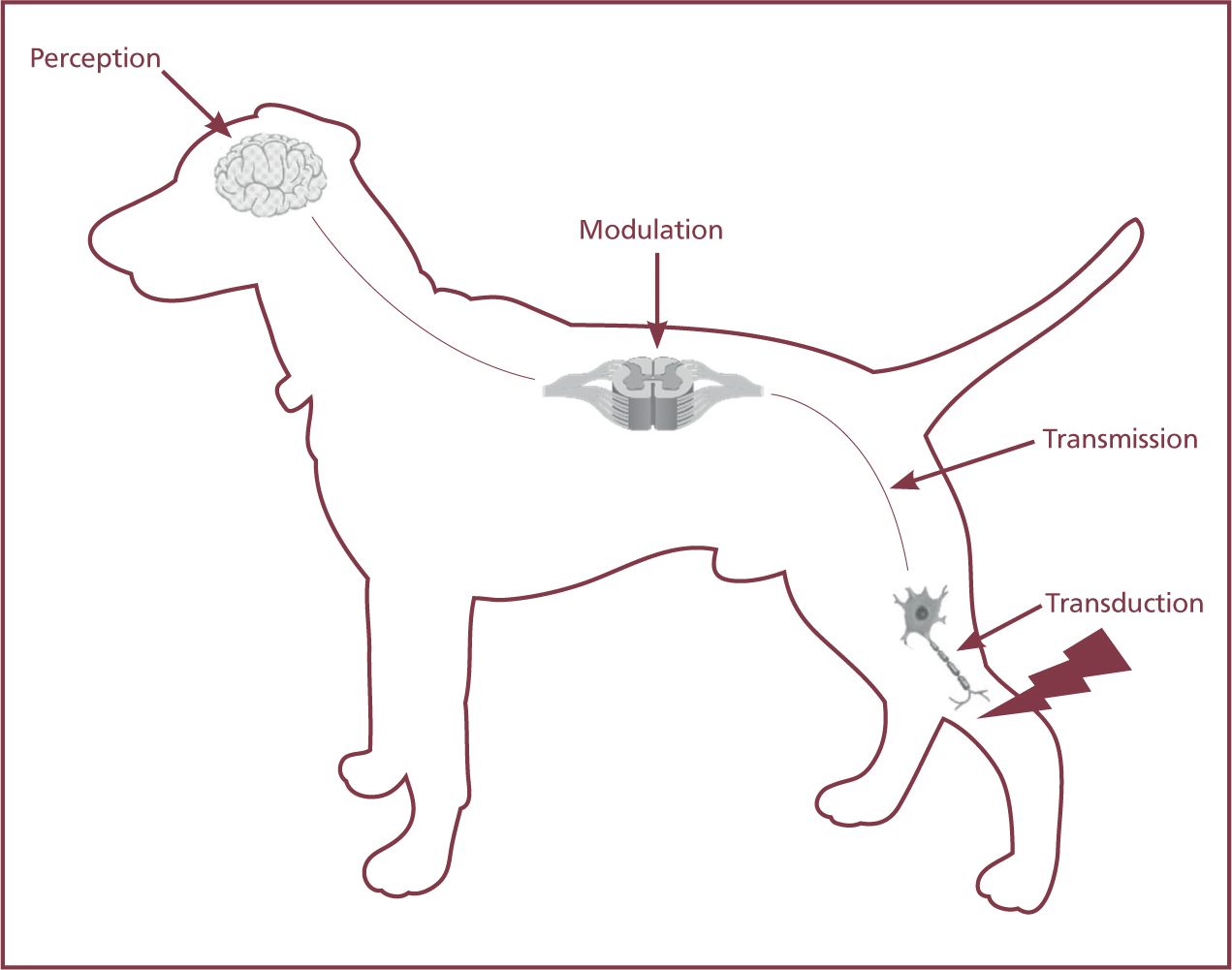
Once activated, a nerve impulse is conducted from the site of the noxious stimuli through to the dorsal horn of the spinal cord, then to the brain in a process known as transduction, transmission, modulation and perception, as described in Figure 3.
The perceived pain is then classified as somatic, visceral, or neuropathic:
- Somatic pain comes from skin and superficial muscles
- Visceral pain is ‘organ pain’ in the abdomen or thorax and is hard to pinpoint
- Neuropathic pain is damage (or malfunction) to the neurons in the central or peripheral nervous system and is a factor in central sensitisation or ‘wind up’ pain.
A multimodal approach to pain is described as using several drugs that work at different sites of the nociceptive pathway, with NSAIDs working at the transduction and modulation stage as seen in Figure 4.
Analgesia is provided by inhibiting the role of prostaglandins to increase nociceptor sensitivity or ‘firing threshold’ in the peripheral tissue.
Through neuromodulation, NSAIDs prevent hyperalgesic ‘wind-up’ sensitisation to pain by decreasing prostaglandin synthesis in the brain and spinal cord (Kerr, 2016). NSAIDs also influence the sensory N-methyl-D-aspartate (NMDA) receptor by inhibiting the production of gluconate (Dugdale, 2010). As an adjunct, NSAIDs work synergistically with other opioids allowing for opioid-sparing (Allweiler, 2013), and potentially a reduction in the opioid dose, dependent side effects.
The analgesic dose is lower than the anti-inflammatory dose, so in the case of chronic pain states the dose may be able to be reduced over time (Dugdale, 2010).
Antipyretic
The inflammatory process releases interleukin-1 (IL-1), a type of cytokine, which is an endogenous pyrogen. These increase the production of prostaglandin in the hypothalamus, elevating the set point for body temperature, eventually causing pyrexia. By inhibiting the production of prostaglandins, this helps to restore the patient's temperature and therefore patient comfort (Dugdale, 2010).
Considerations and contraindications
There are many known side effects of NSAIDs which are generally associated with the inhibition of COX-1, but as NSAIDs inhibit prostaglandin synthesis overall, their use will have subclinical or mild adverse effects on organ systems throughout the body. It is therefore crucial to understand their appropriate use and their specific considerations in the individual patient, especially as they cannot be antagonised. Some contraindications are:
- GI ulcers or disease
- Renal or hepatic dysfunction or impairment
- Hypovolaemia or patients with heart disease that may contribute to poor tissue perfusion
- Patients with known coagulopathies or those at risk of bleeding (e.g. splenectomy)
- With other NSAIDs or steroids.
Approximately 55% of dogs may develop adverse effects where vomiting and diarrhoea are most seen (Monteiro-Steagall et al, 2013).
Gastrointestinal effects
The good ‘housekeeping’ prostaglandins (PGI2 and PGE2) in the GI tract have a number of homeostatic roles; they regulate the gastric blood flow and produce the protective mucus and bicarbonate layer in the stomach, they modulate gastric acids and enzyme secretion, promote gut motility and they repair the epithelial cells, reducing the risk of gastric ulcers. Although COX-2 is the primary enzyme in the inflammatory response, it does, however, play a role in ulcer healing (Dugdale, 2010). Unfortunately, GI side effects are caused by potentially inhibiting these with NSAIDs.
NSAIDs that are non-selective to either COX-1 or COX-2 enzymes are more likely to induce adverse effects such as vomiting, diarrhoea, anorexia and abdominal pain, which can cause gastric ulceration, with or without haematemesis or melaena (Monteiro-Steagall et al, 2013). These effects are increased when high doses are administered, when taken for a prolonged period, if the patient has pre-existing GI disease, and when multiple NSAIDs or concurrent corticosteroids are being administered. Signs of adverse GI side effects are usually seen within 2–4 weeks (Hampshire et al, 2004), but it has been seen to occur at any point before then.
NSAID admission should be stopped in patients that show GI side effects. They should be allowed to fully recover before starting another type of NSAID, with a rest period of least a week (Kerr, 2016).
In patients that have developed signs of a gastric ulcer or are at high risk of getting one, prophylactic gastroprotectants can be used as shown in Table 3, however routine administration of gastroprotectants is not advised (Kilpatrick, 2020).
Table 3. Drug options for the treatment of gastric ulcer
| Drug | Mechanism of action |
|---|---|
| Sucralfate | Dissociates the ions in the acidic stomach environment forming a protective barrier over ulcer sites |
| Omeprazole | A proton pump inhibitor (PPI) which reduces the amount of stomach acid produced by the parietal cells in the stomach through the H⁺/K⁺ ATPase enzyme. It is 10 times more potent than cimetidine |
| Famotidine | A H2 histamine receptor antagonist which decreases the production of stomach acid by the parietal cells. It is 7.5 times more potent than ranitidine, and 20 times more potent than cimetidine |
| Ranitidine | A H2 histamine receptor antagonist which decreases the production of stomach acid by the parietal cells. It is more potent than cimetidine, but it has lower bioavailability (50%) |
Renal
COX-1 and COX-2 isoforms are expressed in the kidney, so unfortunately even the more desirable COX-2 selective drugs can produce adverse renal effects.
Prostaglandins are formed in the kidney to maintain renal blood flow (RBF) when it is reduced as a result of hypovolaemia, dehydration or hypotension, by vasodilating the afferent arterioles which supply the nephron of the kidney. Prostaglandins are also involved in the autoregulation of renin secretion (affecting blood pressure and vasodepressor activity) and tubular transport in the kidney (allowing reabsorption and filtration of ions).
Under anaesthesia, it is not uncommon for mild-moderate periods of hypotension to occur, and with the use of NSAIDs this protective mechanism of the prostaglandins maintaining RBF is affected. In patients that have reduced renal or cardiovascular dysfunction that may affect RBF, there is a risk of an acute kidney injury/failure because of the lack of blood flow at the time of anaesthesia. The medulla in the kidney is the most susceptible to ischaemic damage from a lack of perfusion (Dugdale, 2010).
If NSAIDs are given pre-operatively, blood pressure monitoring and the administration of intravenous (IV) fluid therapy should be performed and administered. Even if they are not administered alongside anaesthesia, patients should be euvolaemic and normotensive for their use.
In cats with chronic kidney disease, meloxicam can be safely administered if they are clinically stable (Gowan et al, 2011). Robenacoxib has been shown to be safe for use in healthy cats and dogs that receive antihypertensive drugs and loop diuretics (Kongara and Chambers, 2018).
Hepatic
As previously mentioned, most NSAIDs are metabolised by the liver. There is both phase 1 and phase 2 hepatic metabolic pathways which are important:
- Phase 1 — conversion of the drugs to a more polar molecule through oxidation, reduction or hydrolysis
- Phase 2 — conjugation of the drug or its metabolites through glucuronidation, acylation, sulfate, or glycine so they can be utilised and excreted by the body.
The use of NSAIDs in patients with renal and hepatic dysfunction or disease is therefore contraindicated because of reduced metabolism and elimination of the drug and its metabolites. Excretions of metabolites are mainly in the urine; however, some may also be excreted in bile which means there may be some enterohepatic recirculation (Dugdale, 2010) where the drug goes back into circulation after being absorbed by the intestine.
As the liver produces most of the circulating proteins, they should be used with care in patients that are hypoproteinaemic because of NSAIDs being highly protein bound.
Antithrombotic
Thromboxane is released from platelets which helps them clump together (aggregation) during coagulation. Inhibiting COX-1 will reduce the production of thromboxane which can lead to prolonged bleeding (Kerr, 2016). Non-selective COX inhibiting drugs should not be used perioperatively, and any administration of these drugs should be withdrawn at least 2 weeks prior to surgery.
The short-term use of COX-2 selective NSAIDS does not have a significant impact on platelet function in healthy dogs (Kerr, 2016), however after 7 days of administration there are detectable alterations in platelet function (Mullins et al, 2012).
Timing of administration during general anaesthesia
The American Animal Hospital Association 2020 AAHA Anesthesia and Monitoring Guidelines for Dogs and Cats recommends NSAID use either before anaesthesia (prior to admission), as part of premedication, or administered in the recovery phase (Grubb et al, 2020).
The effectiveness of NSAIDs being able to reduce peripheral and central sensitisation is greater when given preemptively before pain or inflammation occurs, as changes in the nociceptive pathway form once pain has been experienced, which may make it more difficult to manage analgesia effectively (Cracknell, 2011). When NSAIDs have been given after tissue damage it stops new prostaglandins forming, but cannot break down those already formed, the half-life however of prostaglandins is between 10 seconds to 5 minutes (Pelley, 2012).
Washout period from steroids and NSAIDs
If the patient has developed side effects with one NSAID and treatment is started with another, there should be a wash out period of 7 days (or 3–4 times the half-life of the original NSAID). If the patient continues to have adverse side effects after a different NSAID has been given, then they are NSAID intolerant and treatment should stop.
Corticosteroids act at the level of the phospholipid (PLA2) layer, higher than the level where arachidonic acid promotes formation of prostaglandins. Therefore, their concurrent use can increase the chances of toxicity (Grint, 2021).
Concurrent drug considerations
As NSAIDs are highly protein bound they may compete and displace other highly protein bound drugs and alter their effects, e.g. phenobarbital. NSAIDs should not be used alongside drugs that modify renal prostaglandins, such as diuretics and angiotensin-converting enzyme (ACE) inhibitors.
Toxicities
The long-term administration of NSAIDs can cause hepatotoxicity, which is indicated when liver enzymes are increased three-to fourfold (Albino, 2015). Patients should have normal renal and hepatic function established before NSAIDs are used.
Cats have a deficiency in the glucuronyl transferase enzyme and therefore have limited ability to metabolise drugs through glucuronidation, leading to a prolonged half-life and a buildup of toxic metabolites, which is why paracetamol is absolutely contraindicated in cats. It may also cause the half-life of some NSAIDs to be prolonged, which can contribute to the risk of toxicity if dose and frequency is not tailored specifically to the species. This is less of a problem with meloxicam because of its clearance by oxidative enzymes (Sparkes et al, 2010).
Ibuprofen is a non-selective COX inhibitor and in dogs has been shown to have serious GI and renal toxicity even at doses that are unable to provide any anti-inflammatory effects (Hanson and Maddison, 2008).
Other pharmacological effects
There are some other pharmacological effects of NSAIDs:
- Antineoplastic effects
- Endotoxin effects
- Reproductive effects. These are not covered within the remit of this article.
Common NSAIDs in veterinary medicine
Carprofen Carprofen is a COX-2 selective NSAID that comes in an injectable and oral formulation. The injectable form can be given subcutaneously (SC) or IV and has an onset of action in 1 hour, and the oral form has a peak plasma concentration within 1–3 hours. It is licensed for pre-operative use in cats and dogs, and long-term in dogs with chronic pain. Repeated administration in cats is not recommended because of the prolonged half-life (20 hours in cats compared with 10 hours in dogs), and it is therefore licensed only for one single peri-operative dose in cats (Kerr, 2016).
Administration of carprofen pre-operatively rather than post-operatively showed a better analgesic effect (Lascelles et al, 1998). Carprofen did not cause clinically relevant renal changes in anaesthetised dogs, even when it was administered before surgery or given to patients with traumainduced alterations in renal function or haemostasis (Bergmann et al, 2005). However, maintaining renal blood flow with fluid therapy and maintaining normovolaemia is still advantageous.
Carprofen also does not alter thromboxane serum levels or have a significant effect on mucosal bleeding times in dogs (Hickford et al, 2001).
It provides a similar level of analgesia compared with meloxicam (Kerr, 2016). Both carprofen and meloxicam have chondroprotective properties (Hanson and Maddison, 2008) by reducing the cartilage metabolism and turnover, therefore it is useful in patients with destructive joint disorders.
Meloxicam
Meloxicam is a COX-2 selective NSAID for use in both cats and dogs. It comes in an injectable and in an oral formulation, in both tablet and syrup form with two different concentrations. The injectable form can be given SC or IV and has an onset of action in 1 hour, and the oral form has a peak plasma concentration within 1–3 hours. It is licensed for pre-operative use in cats and dogs and can also be given postoperatively. It has a long half-life, so it is given only once a day (20–30 hour half-life in dogs, 11–21 hour half-life in cats) and it provides analgesia for 24 hours. Dosages vary depending on its short-term or long-term use between species.
In dogs, no adverse effects in renal function have been seen when administered pre-operatively, even during times of mild hypotension where the mean blood pressure was 54±7 mmHg (Boström et al, 2006).
As mentioned previously, meloxicam is chondroprotective.
Robenacoxib
Robenacoxib is a highly selective COX-2 NSAID licensed for use in both cats and dogs in cases of chronic osteoarthritis, and peri-operative orthopaedic and soft tissue pain. It comes in an injectable formulation for SC administration, and an oral form that has better bioavailability in fasted animals.
It is superior to meloxicam in soft tissue pain in cats (Kamata et al, 2012) and has a similar effect to carprofen and meloxicam in dogs when used after orthopaedic or soft tissue surgery (Edamura et al, 2012; Gruet et al, 2013).
Firocoxib
Firocoxib is a highly selective COX-2 NSAID licensed for osteoarthritis and post-surgical pain in dogs. It can be given 2 hours before surgery (Pelligand and Elliot, 2017). It comes in an oral formulation only and has a good safety profile. It is superior to carprofen in dogs with osteoarthritis (Pollmeier et al, 2006).
Mavacoxib
Mavacoxib is a highly selective COX-2 NSAID licensed for the use in dogs with osteoarthritis pain. The dosage periods are long because of the half-life of 8–39 days (Kerr, 2016).
Grapiprant
Grapiprant is one of the newest NSAIDs available in the veterinary market and it is classified as a pirpant, a non-COX-inhibiting prostaglandin receptor antagonist. Its action is at the specific G protein-coupled eicosanoid (EP4) prostaglandin receptor (PGE2) as seen in Figure 2, which is responsible for osteoarthritis pain and inflammation. This means it does not interfere with other prostanoids and their homeostatic actions (Kirkby Shaw et al, 2015).
It is licensed for use in dogs with osteoarthritis. It is not licensed for peri-operative pain, unless of course the patient is already receiving this drug before surgery (Gurney, 2020).
Despite its specific receptor targeting and mechanism of action, it is still contraindicated for use alongside other NSAIDs and steroids. Vomiting has been reported in dogs (Rausch-Derra et al, 2016).
Paracetamol
It is worth mentioning where paracetamol sits when discussing NSAIDs. It has been described as a weak NSAID without any significant anti-inflammatory properties, however, it may act as a COX-3 inhibitor (which is a splice variant of COX-1) within the central nervous system (Dugdale, 2010). The COX-3 enzymes are responsible for regulating fever and some pain responses (Schwab et al, 2003). Analgesia is provided through the activation of descending inhibitory serotonergic pathways as the metabolite is an endogenous cannabinoid (Dugdale, 2010).
It can be given by IV and oral routes in dogs and is absolutely contraindicated in cats because of their inability to metabolise and excrete the drug through glucuronidation. Paracetamol administration in cats can cause methaemoglobinaemia leading to haemolysis, and liver failure is also seen.
Conclusion
Despite their common use in veterinary medicine to manage pain and inflammation, NSAID use does not come without potential side effects. Understanding the individual pharmacodynamic and pharmacokinetic properties may make their use safer in veterinary patients.
KEY POINTS
- Non-steroidal anti-inflammatory drugs (NSAIDs) reduce inflammation by inhibiting the formation of the inflammatory mediator prostaglandin.
- Most of the NSAIDs commonly used in veterinary medicine are selectively COX-2 inhibitors (the ‘bad prostaglandin’), however, they may still have an effect on the ‘good housekeeping’ COX-1 pathway for prostaglandins.
- NSAIDs work at the transduction and modulation part of the nociceptive pain pathway.
- The administration of NSAIDs should be stopped if there are any unwanted side effects, most commonly vomiting and diarrhoea.
- Concurrent use of NSAIDs and corticosteroids is not recommended as this increases the chance of toxicity and negative side effects.