Pain should always be treated to inhibit its deleterious effects, however, analgesia is not benign and does carry some risk and potential complications. A full understanding of the physiological responses to pain and the positive and negative responses to analgesia is required by veterinary nurses treating neonates in pain. In neonates these responses can differ greatly to adults, and nursing techniques should be adapted to encompass these requirements. In addition, unmanaged painful experience, especially when the nervous system is developing, may have a permanent negative impact on the animal (Mathews, 2008). Studies suggest that infants retain a ‘memory’ of a previous painful experience and their response to a subsequent painful stimulus is altered as this memory makes them more fearful (Taddio et al, 1997). There are many individual differences in reactions to pain; these can depend on species, temperament, gender and age (Taddio et al, 1997). Generally younger animals, particularly neonates, are more reactive to pain than adults, due to a lower threshold for pain (Pascoe, 2000).
Due to important physiological maturation changes that occur during the first 6 months of life (the paediatric period) further demarcation is used: neonatal (0–2 weeks); infant (2–6 weeks); weanling (6–12 weeks); and juvenile (3–6 months). Vast metabolic changes occur during these periods of maturation. Animals between 3 and 6 months of age require adult dosing regimens to achieve sufficient analgesia (Mathews, 2008).
Drug distribution and metabolism
It is important to remember when administering pharmaceuticals to neonates that drug distribution does differ from that of adults. This is due to differences in body composition (lower body fat stores and plasma albumin concentrations, higher percentage of total body water and a poorly developed blood–brain barrier). These differences mean that drug dosages may be required to be reduced by up to 50% of the adult per kg bodyweight dose, and the frequency of administration may also need to be reduced from that used in an adult. It is important to remember that renal and hepatic functions are not yet mature, and will therefore delay drug clearance. The use of NSAIDs in patients under 12 weeks is discouraged (no drug is licensed for use under 12 weeks of age), and adherence to the product’s data sheet is recommended.
Monitoring of body temperature needs to occur regularly (if the temperature is normal three times a day, if the temperature is outside of normal range every hour to see if the measures put in place are working), safe supplemental heat needs to be provided in the majority of cases because neonates are not as good at regulating their body temperature as adults. Metabolism of analgesic pharmaceuticals can be reduced if the animal is hypothermic (Luks et al, 1998). Inhibition of the absorption of some drugs can occur where medications are given orally; the presence of milk in the stomach may inhibit absorption, potentially resulting in lower levels of the drug in the blood (Mathews, 2008).
In addition, any patient under 12 weeks of age is considered at a higher risk during any anaesthetic event that may be required. This is because they possess little cardiac reserve, being more dependent on heart rate for their cardiac output. These patients have an increased oxygen requirement and very small airways making for an increased overall risk of hypoxia. In cases where anaesthesia is required (for example to place a central line, or blood sample), hypoglycaemia during the pre-starvation period can occur and signs (disorientation, lethargy, shaking, tremors, seizures, coma) should monitored. Food should only be withdrawn for 3 hours, provision of glucose intravenously (via fluid therapy) should be considered as neonates have very limited glucose reserves.
Pharmaceutical classes of analgesia
There are seven main classes of analgesia, and combination therapy or multimodal therapy should be considered in order to allow reduction in doses of the agents used (balanced analgesia). This has the benefit of enabling reduced doses, which aid in the reduction of both severity and frequency of undesired side effects; commonly used dose rates are listed in Table 1. The main classes of analgesia are:
| Drug | Species | Dose (mg/kg) (lower dosages in patients less than 4 weeks of age) | Routes of administration (SC suggested for patients less than 4 weeks of age) | Interval (hours) |
|---|---|---|---|---|
| Mild to moderate pain Morphine | Dog | 0.1–0.5 | IM, SC, extremely slowly IV | 1–4 |
| 0.05+ | IV, SC per hour | CRI | ||
| 0.25+ | PO titrate to effect | 4–8 (8) | ||
| Cat | 0.05–1 | IM, SC | 1–4 | |
| 0.025+ | IV, SC per hour | CRI | ||
| 0.25+ | PO titrate to effect | 4–6 (8) | ||
| Methadone | Cat and dog | 0.1–0.5 | IV, IM, SC | 1–4 |
| Fentanyl | Cat and Dog | 0.002–0.001 |
IV loading |
0.5–1 |
| Butorphanol | Dog | 0.1–0.2 | IV, IM, SC | 1–4 |
| Cat | 0.1–0.2 (to effect) | IV, IM, SC | 1–4 | |
| Dog and cat | 0.05–0.01+ | IV, SC per hour | CRI | |
| Buprenorphine | Dog and cat | 0.005–0.01 | IM, IV | ~6 |
IM, intramuscular; SC, subcutaneous; IV, intravenous; PO, per os
Intravenous fluid therapy
Hydration levels of the neonate patient should be noted as dehydration and shock will reduce peripheral circulation and thus will affect absorption of any medications administered subcutaneously. Fluid support is, therefore, important, however, neonates are more sensitive to fluid overload than adult animals as their kidneys are immature and unable to ‘work’ at full capacity in filtration and sodium/potassium balance. In addition, as neonates have reduced hepatic function they may be unable to metabolize lactate found in Hartmanns solution into bicarbonate (England and Russo, 2011). Basic fluid rates from 5 to 10 ml/kg/hour are usually sufficient (Boag and Hughes, 2011), and monitoring of fluid balance should occur. Intravenous access can be very difficult in critical patients and if a standard IV catheter is not an option, intraosseous fluids are an effective next choice. Overthe-needle catheters can be placed into the jugular vein in emergency situations (Figure 1). Intravenous access for the administration of pharmaceuticals, fluids and for blood sampling is vital. Fluids containing 2.5 or 5.0 % dextrose may be necessary for these patients, as neonates are prone to hypoglycaemia (Figure 2). Use of continuous rate infusions alongside the intravenous fluid therapy can be beneficial in critical cases of any age, as doses can be tapered to effect more proficiently, with lower doses being given rather than bolus which will peak and trough.


Monitoring of analgesic outcomes
Physiological changes induced by pain should be closely monitored and these include: tachycardia, increased blood pressure, changes in respiratory pattern, panting, pyrexia, salivation, shaking, shivering, inability to rest or sleep and pupillary dilation. These parameters need to be monitored where possible before the pain process has begun (baseline), and before and after any analgesia is administered in order to judge efficacy. Pain scoring systems commonly used may not be suitable for neonates as they are unable to display the behaviours that are traditionally scored (ability to move, groom, urinate, defecate), therefore physiological changes should be closely monitored.
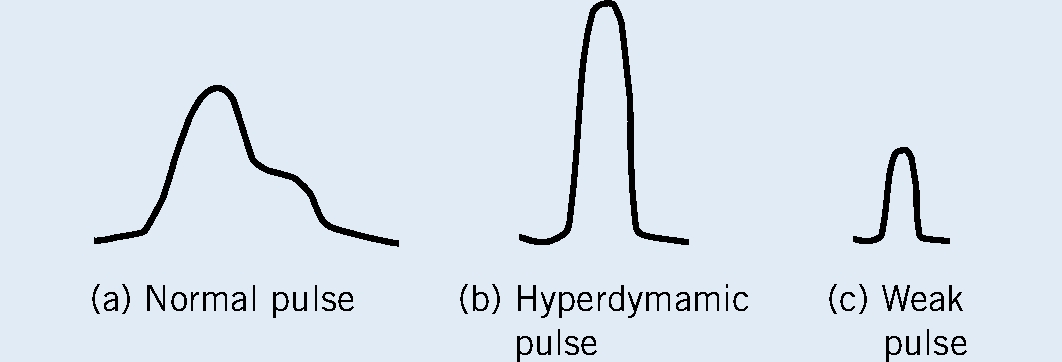
Pulse character is one of the most important parameters to monitor, as it provides an assessment of perfusion status, pain level, stress and shock. In the majority of cases the nursing hospitalization/ monitoring sheets need to be adapted for critical patients, in order for these parameters to be recorded. The pulse should be carefully palpated to allow assessment of both height or amplitude (to assertain whether the blood pressure is strong or weak) and the width or duration of the pulse. A normovolaemic animal in pain will have a slightly taller and narrower pulse profile than a resting animal (Boag and Hughes, 2011). A mental image of the pulse profile can be made; comparison can aid in identifying/indicate improvement or deterioration of the patient (Figure 3).
Knowing what is normal is also vital so that an abnormal temperature, and associated problems, can be easily recognized; the normal rectal temperature for the first week after birth should be 32–34°C. Breathing should always be regular and even, with a normal rate for a neonate being between 15–40 breaths per minute. There should be no excessive noise, if there is this can indicate fluid in the lungs. Daily weighing of the neonate should see a 5–10% increase per day (Ackerman, 2009).
Nursing techniques
Patient comfort can be greatly improved by reducing the number of painful events to which the neonate is subjected. An increase in technical proficiency can aid in preventing pain. For example, if several blood biochemistry and haematological parameters need to be closely monitored, grouping them together to reduce the number of venipunctures would be beneficial, or placing a central line will also be positive for the animal, where serial blood sampling can occur through the central line along with blood pressure measurement. Use of topical local analgesics such as EMLA (eutectic mixture of local anaesthetics) cream may prove to be very useful. Reducing the number of times that the animal is removed from its warm bed or oxygen-rich environment (if receiving oxygen) is another example of how good nursing planning can greatly affect the patient. Moving a neonate patient needs to be done very gently as the sudden change in orientation can lead to a redistribution of circulating blood volume and can cause ‘shock’, which they might not be able to survive (Chandler and Middlecote, 2012).
All medications need to be accurately dosed. It is best to use 1 ml syringes or 100 IU syringes. Some medications can be diluted down, in order to be very specific with dosing, but for this to be done the drug needs to be water-miscible and thoroughly mixed.
It should be remembered that even though the condition of hospitalized neonates is potentially critical, basic puppy/kitten care is still required. Stimulation of urination and defaecation still needs to occur every 2 hours, especially if the animal is receiving intravenous fluids. An environmental temperature of 30–33oC should be maintained for critical patients in the first 24 hours, and then they should be maintained at 26-30oC for 4–5 days, being careful to ensure no draughts. In order to minimize heat loss clipping of any hair needs to be kept to a minimum, and the use of surgical spirit to swab the skin, such as may be required when analgesia is administered, should also be used minimally. Although small points these can mean the difference between life and death in a critical neonate.
Conclusions
There is little information on the use of analgesia in neonates; understanding of the unique physiology of these animals is required when considering the selection of analgesia. Most veterinary texts advocate the use of opioids as the safest form of analgesia for this population of animals. Starting at lower doses and increasing to effect is recommended, so close monitoring is required.
Close monitoring of neonate patient is vital in identifying any effects of treatments and nursing care. The maintenance of blood glucose levels and body temperature are vital in the maintenance of life, but are often over looked.
