Pharmacokinetics is the study of the processes of drug absorption, distribution and elimination, either by metabolism or excretion. Detailed pharmacokinetic studies quantify these events and their time course.
Drug absorption
The majority of drugs do not exert their effects in the compartment into which they are delivered, e.g. the gastrointestinal tract for drugs given orally or the plasma for drugs given intravenously. Drugs need to cross lipid cell membranes in order to reach their site of action and this can be achieved in a number of ways:
- Simple diffusion: drugs move from a high to a low concentration by diffusion, a passive process that does not require energy.
- Non-ionic diffusion: most drugs are either weak acids or weak bases, which means that when they are dissolved in water they will either give up a hydrogen ion and become ionized acids, or accept a hydrogen ion from the water and become ionized bases. The ionized form has an electric charge and in this form it is unable to cross a lipid membrane.
(a weak acid will give up a hydrogen ion in water and become ionized)
(a weak base will accept a hydrogen ion in water and become ionized)
When in solution the ionized form of the drug is present in equilibration with the unionized form, how much of the drug changes to the ionized form depends on the pKa of the drug and the pH of the solution. The pKa of the drug is related to the equilibrium that the drug has with its ionized form and is the pH of the solution at which 50% of the drug will be ionized and 50% will be unionized. Therefore when the pH of the solution is the same as the pKa of the drug then 50% of the drug will be ionized and 50% of the drug will be unionized.
Clinical relevance: only the unionized from of the drug is lipid soluble and can cross cell membranes to exert a pharmacological effect.
A good clinical example relates to the effectiveness of local anaesthetics injected into inflamed tissue. Local anaesthetics are weak bases and therefore become more ionized in inflamed tissue because it is more acidic.
This reduces the effectiveness of local anaesthetics in inflamed tissue because uptake of the drug across nerve fibre membranes is reduced, due to the higher proportion of the injected drug becoming ionized.
- Carrier transport: proteins that are present within the cell membrane may act as carriers for the drug and increase uptake of the drug across the cell membrane. Two types of carrier transport are recognized. 1) Active transport where the drug is transported against a diffusion gradient into the cell. This process requires energy. 2) Facilitated diffusion in which the drug molecules move passively across the membrane from a high concentration to a low concentration attached to a carrier molecule. This process does not require energy.
Bioavailability
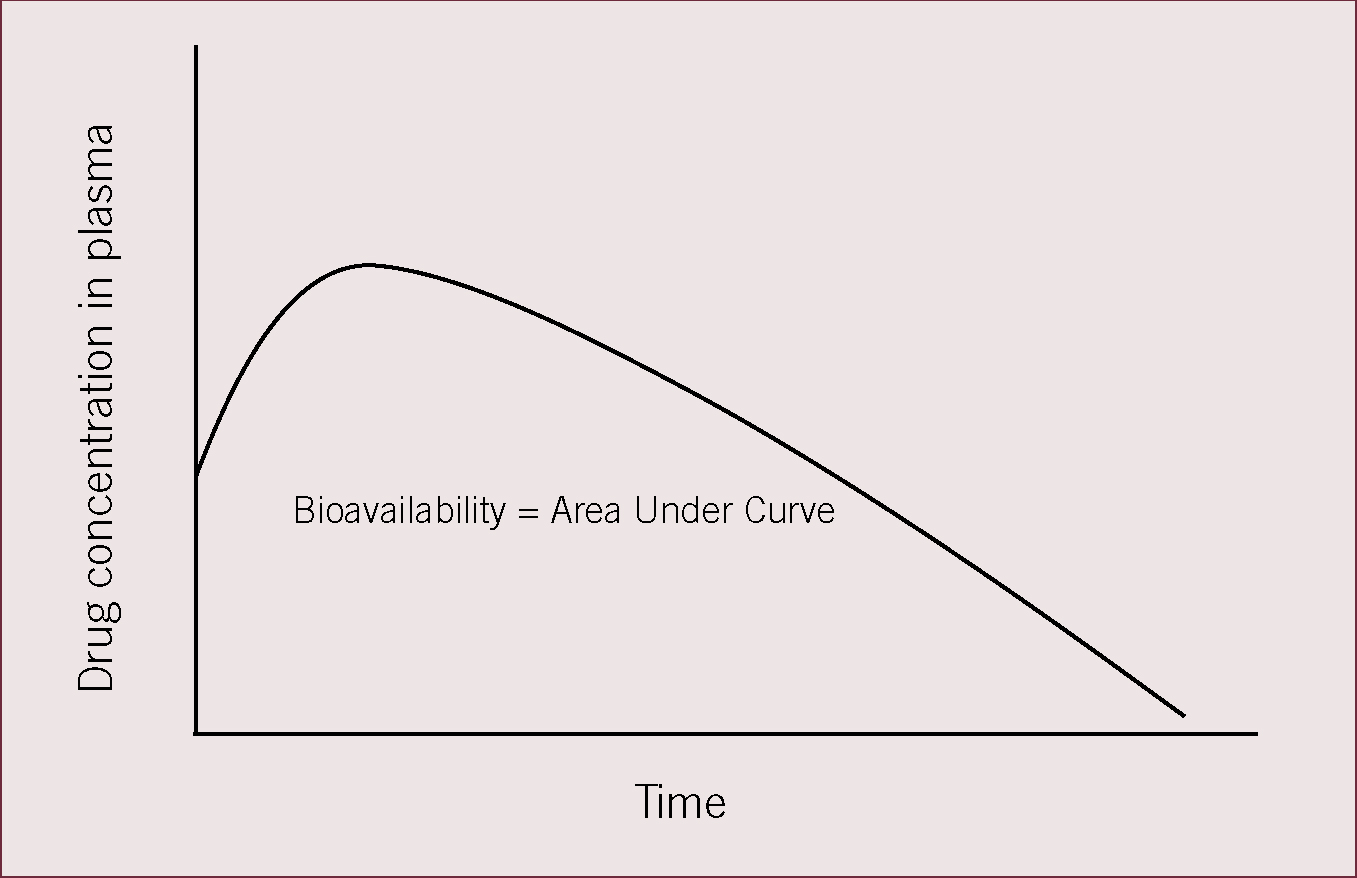
This is one of the principle pharmacokinetic variables used to describe a drug. Bioavailability describes the fraction of an administered dose of unchanged drug that reaches the systemic circulation. When a drug is given intravenously bioavailability is 100%, administration by other routes usually decreases bioavailability due to incomplete absorption or the effects of first pass metabolism. Bioavailability essentially captures two essential features of a drug, namely how fast the drug enters the systemic circulation and how much of the drug enters the systemic circulation. Bioavailability following oral dosing may also be affected by factors such as timing of dosing relative to feeding of the patient and so can be manipulated in the individual patient. Bioavailability must be considered when calculating doses for non-intravenous routes of administration. Oral bioavailability can be calculated by plotting a graph of drug concentration against time (Figure 1), the area under the curve is bioavailability.
Routes of administration
Drugs can be administered by a variety of routes, each with their own advantages and disadvantages. Selection of an appropriate route will depend on the drug, the situation surrounding administration (i.e. is the owner going to administer the drug?), and factors such as desired onset of action or the temperament of the animal. The advantages and disadvantages of different routes of administration are given below.
Oral
Oral administration of drugs is simple, non invasive and cheap because orally administered drugs do not need to be sterile. This route also does not cause pain to the patient and it is a convenient route by which owners can administer drugs to their animals at home. However, absorption of some drugs from the gastrointestinal tract is slower and less consistent than the intravenous route of administration. It also requires the patient to be conscious and able to swallow, which limits its usefulness for the administration of drugs in the peri-operative period. The first-pass effect must also be taken into consideration. Drugs absorbed orally are transported to the general circulation via the liver where a degree of drug metabolism usually occurs before the drug enters the systemic circulation resulting in lower than predicted plasma concentrations of the drug and therefore drug effect. Food and gastrointestinal motility can also influence the rate of drug absorption, which is why the manufacturers' recommendations regarding the timing of oral drug administration relative to feeding must always be adhered to, and explained to owners when drugs are dispensed for administration at home.
Sublingual or buccal
Drug absorption following delivery to the mucous membranes in the mouth is usually rapid due to the good blood supply to the area of absorption, although this will vary according to the physiochemical properties of individual drugs. Not all drugs are well absorbed by this route. The liver is by-passed because the drug is absorbed directly into the systemic circulation, therefore there is no first-pass effect. It is also non-invasive and painless and if the drug is not bitter then animals do not usually find this route aversive. A good example of a drug that can be administered sublingually to cats is buprenorphine. Studies have shown that buprenorphine is well absorbed and efficacious by this route (Robertson et al, 2005) and it avoids pain that would otherwise be associated with repeated intramuscular or subcutaneous administration of the drug in the peri-operative period.
Intravenous
This is the most common route used for the delivery of injectable anaesthetic agents for induction or maintenance of anaesthesia. Continuous rate infusions are also most commonly given intravenously. The drug can be given rapidly and the total dose is delivered into the blood stream so that drug effects are more predictable than other routes of administration. It allows titration of drug dose to drug effect because there is minimal time lag between drug administration and the onset of drug effect, reducing the likelihood of drug overdose. Ideally anaesthetic or analgesic drugs should be given via a pre-placed intravenous catheter to avoid the requirement for repeated needle puncture of the vein and potential trauma. Catheter placement also allows the drug to be given in a more controlled way, reducing the likelihood of drug overdose during induction of anaesthesia.
Intramuscular
Absorption by the intramuscular route is generally more rapid and predictable compared with subcutaneous administration. This route is, therefore, preferred for the administration of drugs in the perioperative period (e.g. premedicants and analgesics) that are often not given intravenously, compared with the subcutaneous route. Intramuscular injection is painful, particularly if large volumes are required and volumes of more than 2 ml are not recommended to be given into a single muscle, it is better to split the dose and divide between two sites. Common sites for intramuscular injection in cats and dogs are the quadriceps, triceps or epaxial muscles. It is important that the animal is adequately restrained to allow the injection to be carried out successfully and that a needle of appropriate gauge and length is selected. Obese animals may require a 1.5 inch needle for injections made into the epaxial muscles to ensure that the drug is not delivered into the subcutaneous fat layer. Choosing the smallest gauge needle that is long enough and allows rapid injection of the drug will reduce pain associated with injection. If a drug is likely to require repeated and frequent administration, for example methadone administration in the post-operative period, then it can be advantageous to opt for the intravenous route to avoid pain. If repeated intramuscular administration is required it is good practice to sequentially rotate the muscles that are injected to avoid one muscle group becoming very sore.
Subcutaneous
This route is less painful and animals tolerate larger drug volumes given subcutaneously better than the intramuscular route. However, the major disadvantage of subcutaneous administration is that the rate of drug absorption is slower and less predictable due to the poorer and more variable blood supply to subcutaneous tissues compared with muscles. This can delay the onset of sedation if premedicant drugs are administered by this route, or make the sedative effects of premedicants less predictable. Studies have also demonstrated that low doses of buprenorphine (10 μg/kg) are less efficacious given subcutaneously compared with other routes (Giordano et al, 2010). This is hypothesized to be because the buprenorphine is absorbed slowly, resulting in a slow rise in plasma concentration, which is inadequate to drive buprenorphine into the central nervous system where it exerts its effects to cause analgesia. Generally, with the exception of many non steroidal anti-inflammatory drugs, the subcutaneous route is not recommended for the administration of drugs in the peri-operative period.
Transdermal
There is a time lag between patch placement and attainment of adequate plasma concentrations of the drug in order to achieve clinical effect. This limits the usefulness of patches for administration of most anaesthetic drugs, but there has been significant veterinary interest in using transdermal fentanyl and buprenophine patches for prolonged analgesia. The transdermal route is non invasive and painless, avoiding the requirement for repeated injections. However, individual variability in transdermal absorption (Egger et al, 1998) affects the plasma concentrations of the drugs that can be achieved, and therefore drug efficacy in individual patients. Giving analgesic drugs by the transdermal route must be combined with pain assessment in order to ensure that the patches are effective, ideally they should be used as part of a multi-modal analgesia technique.
Inhalation
Drugs are generally rapidly absorbed by this route because of the vascularity of lung tissue encouraging the uptake of inhaled gases into the systemic circulation. There is no first-pass metabolism effect. Volatile anaesthetic agents for maintenance of anaesthesia are delivered by inhalation, usually combined with induction of anaesthesia using an injectable technique. This ensures induction of anaesthesia is rapid and non aversive, while maintenance of anaesthesia with volatile agents is not associated with drug accumulation, so that recovery from anaesthesia is not prolonged.
Distribution of drugs in the body
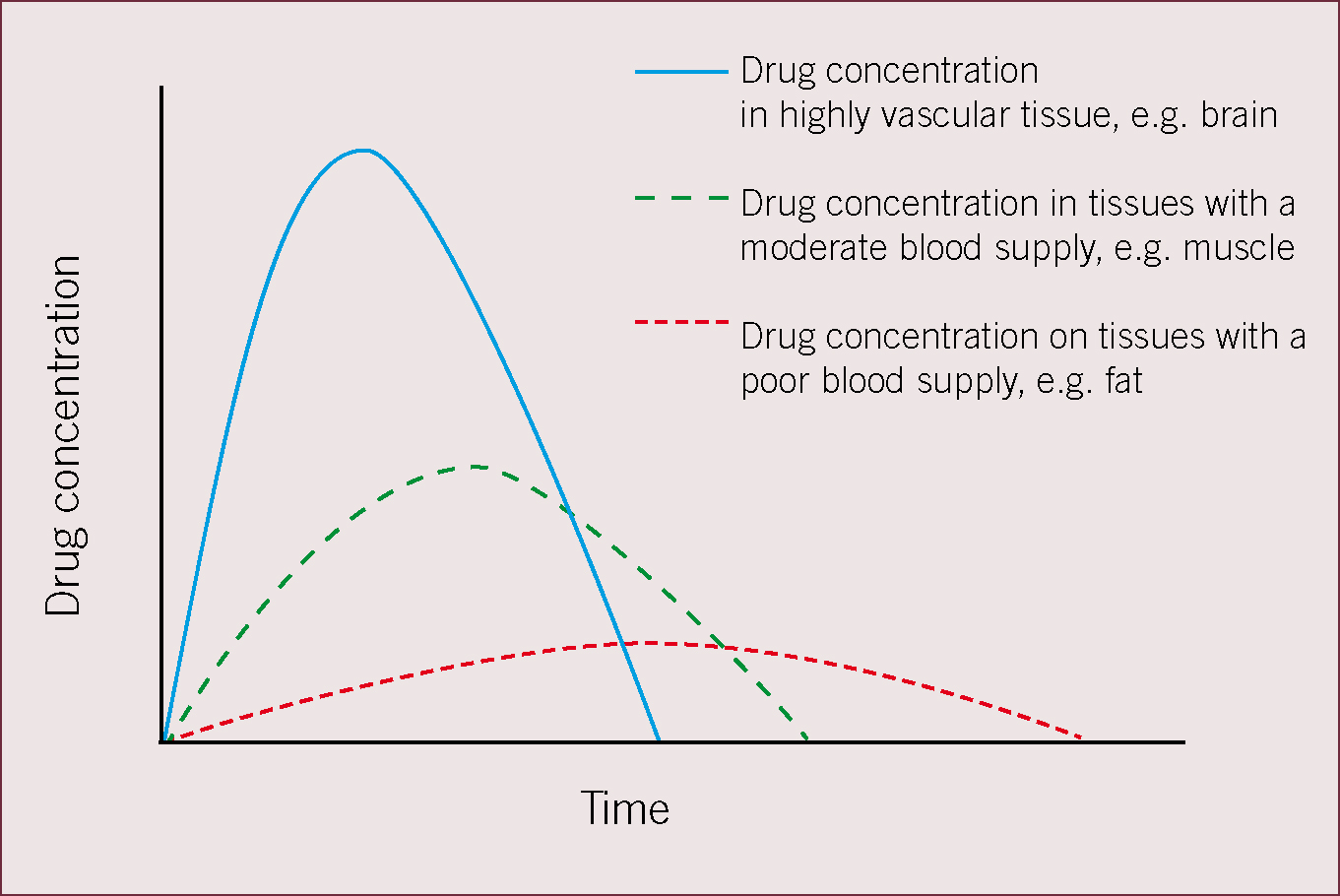
Once the drug is present in the blood stream a number of factors will interplay to cause a reduction in plasma concentration. Elimination by metabolism and excretion will start (see the second article), but at the same time the drug will be distributed to other tissues. Distribution is responsible for the rapid fall in drug concentration after intravenous injection. The rate of drug distribution to other tissues depends on their relative blood supplies. Initial distribution is to tissues with a high blood flow, such as the brain, heart, lungs, hence intravenously injected anaesthetic agents quickly cause induction of anaesthesia. Distribution to tissues that receive a lower blood flow then follows, e.g. muscle tissue, and finally drugs are distributed to poorly vascularized tissues such as fat. This process is shown schematically in Figure 2.
First order kinetics
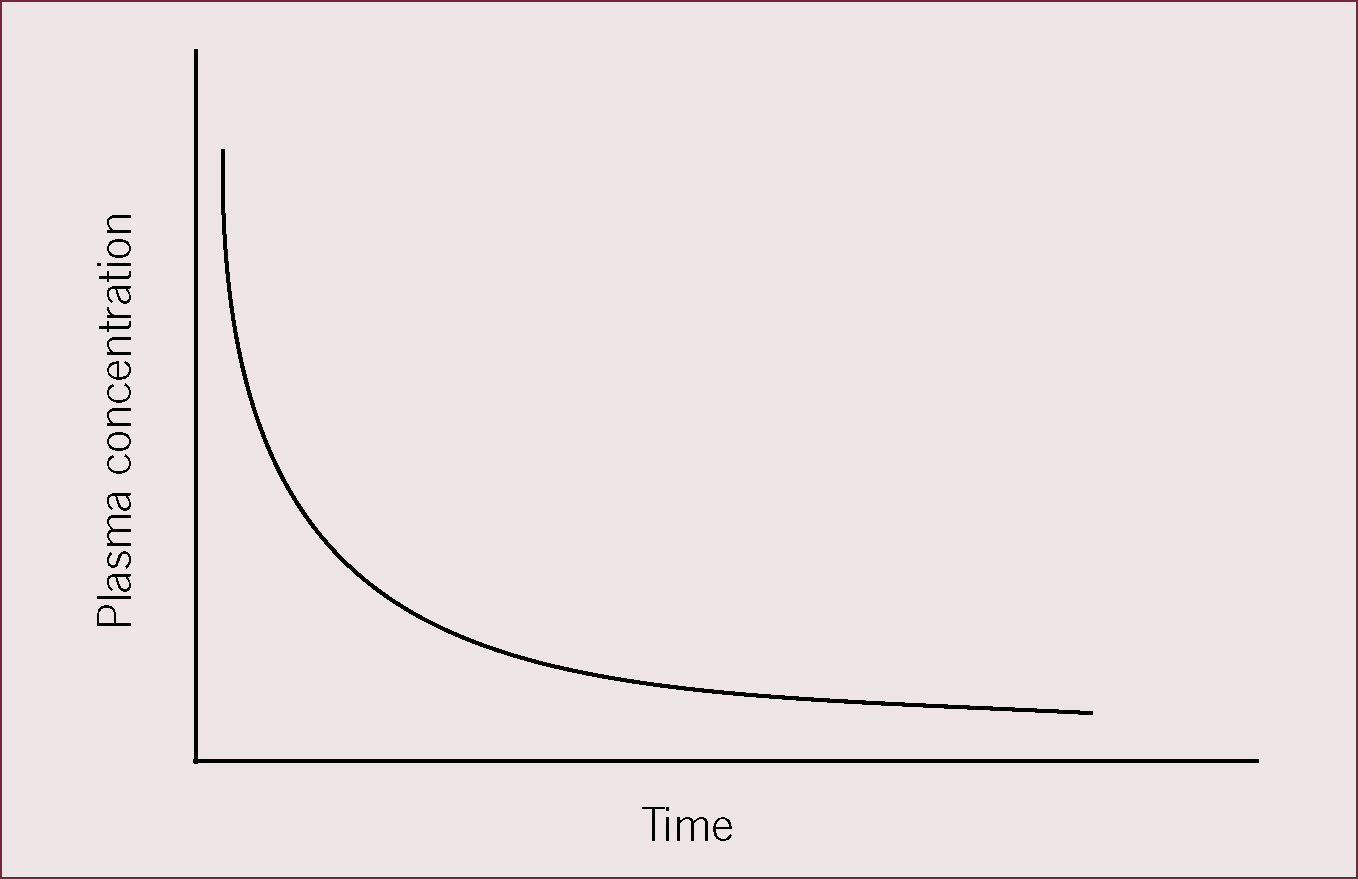
Kinetics describes the fall in plasma concentration against time, representing the processes of distribution, metabolism and elimination. As the rate of fall in plasma concentration slows, distribution contributes less and less to the decline in plasma concentration and elimination from the body becomes the predominant process.
For most drugs the rate of decline of plasma concentration is related to the amount of drug present, a process known as first order kinetics. This is shown schematically in Figure 3, where after intravenous administration of a drug the rate of decline of the plasma concentration is exponential, i.e. proportional to the amount of drug present. The concentration-time curve can be used to derive a number of pharmacokinetic parameters that have clinical relevance for deciding dose and dosing interval.
Volume of distribution (Vd)
The Vd is the theoretical volume that the drug would occupy if all of the body was at the same concentration as the plasma. The Vd gives some indication of how fat soluble the drug is and how well it binds to proteins. Drugs that are highly protein bound generally remain in the plasma and are not widely distributed throughout the body, therefore Vd is relatively small and close to blood volume. In contrast, drugs that are very fat soluble, such as thiopental, are able to cross cell membranes to enter intracellular fluid. Therefore Vd is close to total body water. Although Vd is a theoretical construct, in that it can be much greater than total body water, it is a useful concept that allows the speed of awakening to be understood after the intravenous administration of anaesthetic agents. For example, the metabolism of thiopental is relatively slow, however, following intravenous administration of a single dose of thiopental and induction of anaesthesia, awakening is relatively rapid and not determined by drug metabolism. The Vd of thiopental is relatively large, therefore, following intravenous administration plasma concentration of the drug falls rapidly as the drug is widely distributed throughout the body. Once the plasma concentration has fallen below a critical concentration return of consciousness will occur.
Conclusions
This article focuses on key elements of pharmacokinetics, namely drug absorption and distribution. The second article will describe drug metabolism and elimination as well as incorporating important pharmacodynamic principles.

