Lavage is one of the most important parts of wound management, and there are multiple reasons why it is beneficial and is thought to help to optimise wound healing. The main aim of wound lavage, or as it also termed irrigation, is to loosen foreign material within a wound or necrotic tissue, while diluting any contaminants, such as bacterial load that may be present (Aldridge, 2013). Hussey and Bagg (2011) stated that the initial 6 hours following injury is known as the ‘golden period’ and if lavage is not carried out in this period, the risks of infection are considerably increased.
Analgesia should always be considered before lavage is initiated. If the patient's condition allows, chemical restraint should be considered to minimise further trauma, reduce further contamination and for analgesia (Aldridge, 2013).
Lavage rehydrates any potential necrotic tissue, which can potentially help to preserve some surrounding tissue, and aid in the removal of any tissue not required by debridement (Kirk, 2014). The process will also reduce the amount of foreign material present if it is done correctly, removing any toxins and cytokines from the wound, and removing any surface debris and bacteria (Anderson, 2012).
Lavage can also help remove any remnants of wound dressings with minimal trauma to surrounding fragile tissue (Anderson, 2012; Chivers, 2010). It should be carried out at every dressing change, not just on the first presentation of the wound. The fluids used should be isotonic and non-toxic to cells. The key principle is to ensure large volumes are used, so that even if all contaminants are not completely removed, they are at least diluted (Anderson 2012).
Biofilms
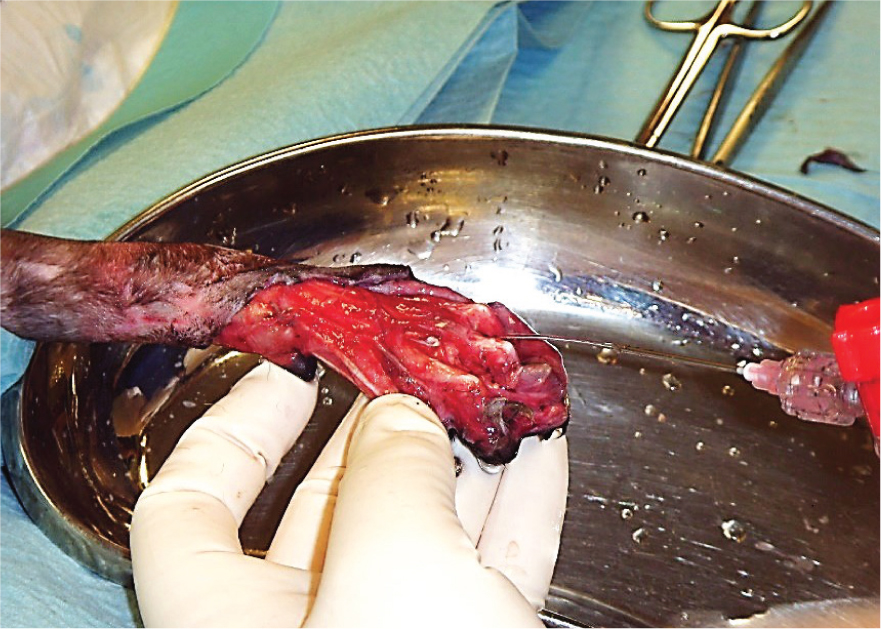
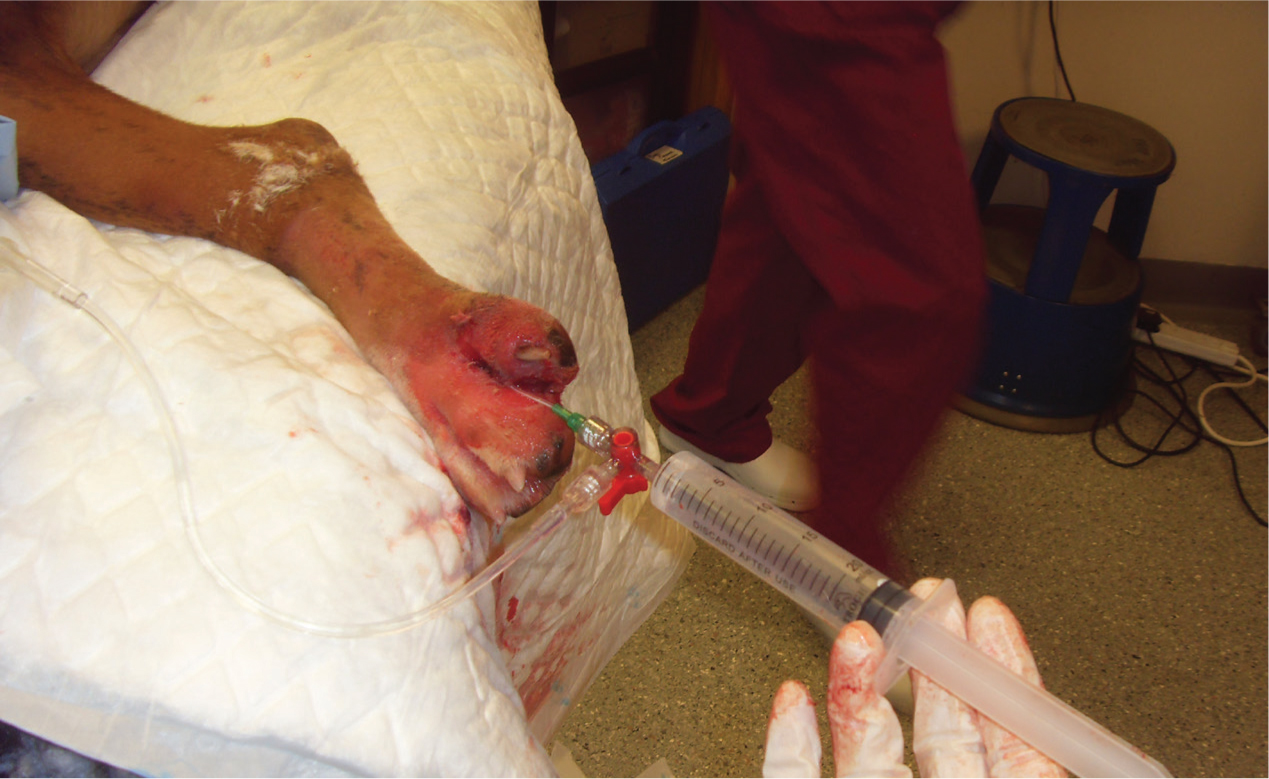
Lavage is fundamental in breaking down bacterial biofilms, which occur when a group of microorganisms (bacteria, fungi, parasites and viruses) attach themselves to a surface to create a colony, alongside debridement. This colony forms itself into a type of ‘shield’, which is a glue-like consistency or slime. The biofilms acts as a barrier to the wound and helps the colony to defend itself against antimicrobial treatments and immune cells (Curtis, 2020). This is partly the reason that some wounds may be difficult to heal, and why persistent infections may keep recurring. Large open wounds, such as the de-gloving injury shown in Figure 1, require thorough lavage because of their traumatic and contaminated nature.
Which solution should I use?
There is much debate on the choice of lavage solutions to use and whether antiseptics should be added to solutions (Aldridge, 2013). Solutions should be non toxic, reduce the number of microorganisms and not cause any reactions (Main, 2008). Normal saline solution is reported to be used in human literature, however most veterinary publications recommend Hartmann's (lactated Ringer's solution) as the wound lavage solution of choice (Aldridge, 2013). The evidence behind this preference is somewhat lacking, however Buffa et al (1997) published a paper that looked at the effects of wound lavage solutions on canine fibroblasts in vitro. The conclusion of this study was that tap water damaged the fibroblasts, normal saline caused cytotoxic effects, but neither buffered saline nor Hartmann's caused any significant fibroblast damage. Despite this evidence, there have not been any further studies conducted to look at whether this is particularly significant in the healing rates and the infection rates in wounds. Conclusions made draw on the evidence that lavage should be carried out with a fluid which is of similar osmotic pressure to that of living cells, isotonic solutions such as normal saline and Hartmann's. Drinking water has been researched in human literature and has the major advantage of being very cheap to use. A study by Svoboda et al (2008), which looked at contaminated animal wounds, showed that there was an identical reduction in bacterial load following lavage with normal saline and drinking water. The isotonic solution TrisEDTA has been shown to have some antibacterial effects by enlarging pore size in antibiotic-resistant Gram-negative bacteria, therefore increasing bacterial kill rates (Anderson, 2012).
The use of antiseptics in lavage solutions can have cytotoxic effects on the cells that are instrumental in wound healing (Aldridge, 2013); cells such as keratinocytes and fibroblasts can be irreparably damaged. Hydrogen peroxide and povidone-iodine reduce cell proliferation and migration of fibroblasts across the wound bed and are dose dependent.
Chlorhexadine and antiseptics containing silver will also reduce proliferation at high concentrations. Morton and Phillips (2012) did however conclude that at lower concentrations they may enhance epithelial growth. If chlorahexadine is to be used, then it should be at very low concentrations (0.05%) as evidence suggests that compared with sterile saline or Hartmann's there is no significant difference in wound contraction or epithelialisation, while it is still able to achieved 100% bacteria kill rates on Staphylococcus intermedius (Aldridge 2013). The skin surrounding the wound can be also cleaned with chlorhexadine at a 0.05% solution or 1% povodone-iodine (Kirk, 2014).
Antibiotics added to solutions are not recommended (Williams, 2009; Aldridge, 2013) as they may cause a reaction in the patient and are unlikely to maintain therapeutic levels for a suitable length of time. In addition, this is a costly exercise and there are concerns about antibiotic resistance becoming an increasing problem in both human and veterinary medicine (Crowley et al, 2007).
The use of soap or surfactants in human medicine for open wounds has been reported to reduce complication rates (Petrisor, 2011). Soap has lipophilic components that block bacterial cell adhesion in a wound, and aids in the removal of these cells. In chronic wounds they are effective at removing denatured proteins (Kaehn, 2009).
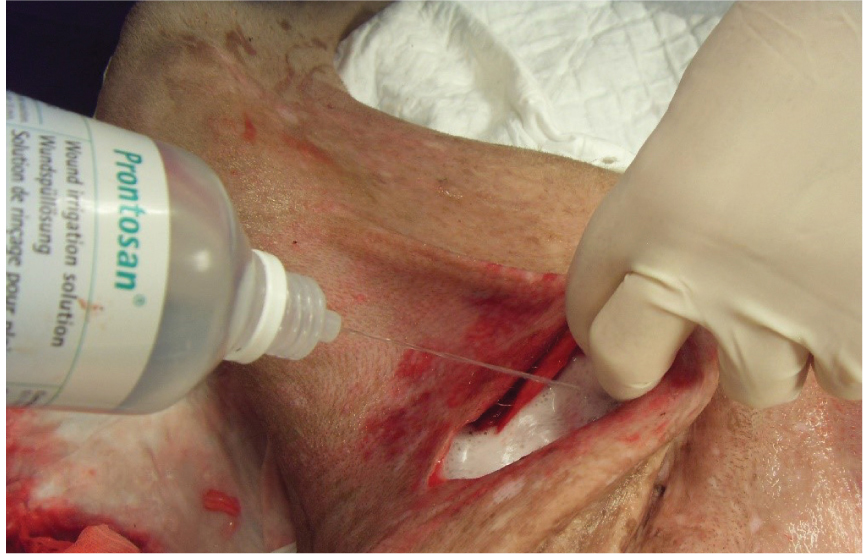
Surfactants such as Prontosan® solution (B Braun) (Figure 2), which is a wound flush, contain a surfactant to break down bacterial biofilms. Bellingeri et al (2016) concluded that Prontosan® solution is superior in efficacy to other wound flushing preparations as it promotes the wound bed preparation, supports the reduction of inflammatory signs and accelerates the healing of vascular leg ulcers as well as pressure ulcers. Lopez-Rojas et al (2017) concluded that Prontosan® solution has high bactericidal activity against multidrug-resistant pathogens. It was also concluded that the bactericidal activity occurs rapidly (1 minute), within a much shorter period of time than that suggested by the manufacturer. An observational investigation into the removal of dressings in human patients showed that the treatment of a variety of skin wounds, in different ages, from paediatric to geriatric age, with a polyhexanide and propyl betaine-based gel in combination with a secondary dressing resulted in significant improvements in the size of the wound, pain at dressing change, and wound characteristics (Durante et al, 2014). Horrocks (2006) concluded that Prontosan® solution was an appropriate alternative to saline for cleaning, moistening and decontaminating encrusted, contaminated and chronic skin wounds. A more recent study by Atkin et al (2020) looking at chronic wounds showed that the use of Prontosan® resulted in improvements to wound bed conditions as early as 2 days after commencing treatment in hard to heal wounds, some of which were of 20 years' duration. Decreasing malodour, exudate, slough and pain was reported across the case series. In addition there was significant reduction in dressing change frequency of 55% in the hard to heal wounds. The product requires 15 minutes' contact time with the wound bed and tissues. It can be used for soaking swabs for packing into deficits (Figure 3), and it is also available as a gel for longer-term use in the wound, and covered by a dressing.
How much solution should be used?
It is believed that the volume of solution used is also important when lavaging wounds. It is recommend either a volume of 500–1000 ml of solution is used or alternatively as a rule 100 ml of solution per centimetre of wound. This in turn means a large amount of fluid is required (Hollis, 2018).
When lavage is complete in the acute stages, it is recommended to take a bacteriology swab to obtain a culture and sensitivity. Debridement is normally required post lavage, and swabs and lavage may therefore continue throughout debridement.
Lavage pressure and equipment
The pressure required for lavage of a wound is controversial and still debated. If lavage is carried out at high pressures this will dislodge surface debris but may also push this debris and bacteria further into the deeper tissue planes, which is detrimental to wound healing. Wounds retaining more than 105 bacteria per gram of tissue will become infected (Moscati et al, 2007), so removal of bacterial burden is essential for wound healing.
If lavage is carried out at low pressures this may not be sufficient to wash debris out of the wound and therefore the aim of the lavage will not have been achieved. Moscati et al (2008) found that pressures of 7 psi or less were inadequate in wound irrigation because of the adhesiveness of contaminants on tissue. The research concluded that higher pressures were more suitable and effective. Gall and Monnet (2010) concluded that pressures of 8 psi or more were damaging to surrounding tissue. High-pressures can infiltrate fluid into surrounding tissues. This can cause oedema and leave the wound more susceptible to infection. It was suggested that use of high pressure should be limited to heavily contaminated wounds where the contraindications are out-weighed by the need to remove the more adhesive bacteria (Edlich et al, 2008).
Pressures of 6–8 psi are normally achieved by using 20–30 ml syringe with an 18 gauge needle attached and considered low pressure (Atiyeh et al, 2009), and Anderson (2012) advised that using this combination of equipment is suitable for most wound types. However this is still debated and Fernandez et al (2004) suggested a 12 ml syringe and 22 gauge needle to create 13 psi is optimum.
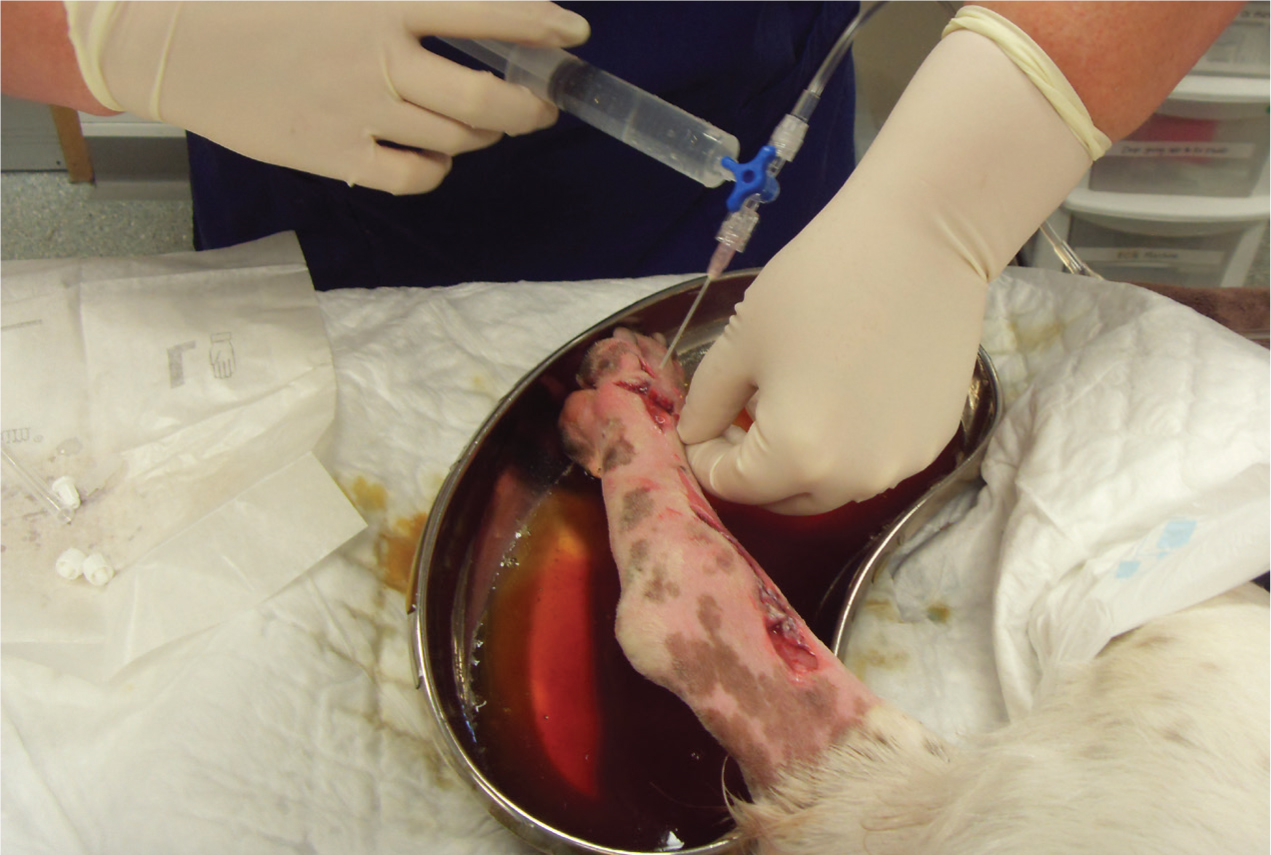
Whichever combination is used, the process will require a firm press on the plunger to create a steady stream to enable the correct psi to be achieved. Using a lavage set, which includes a combination of a 3-way tap, attached to a bag of fluids with an intravenous fluid giving set (Anderson, 2012), can help reduce time and increase efficiency with lavage (Figure 4). Bell (2017) suggested that using a sterile kidney dish to collect excess fluid from flushing can be advantageous to prevent recontamination from the process (Figure 5) or absorbent pads as shown in Figure 6.

Conclusion
Wound lavage is an essential part of management of wounds in veterinary practice. Although there are still some gaps in the knowledge largely because of the lack of evidence in certain aspects of lavage, such as ideal solutions and pressures, we can still be assured that lavage is required to facilitate healing in wounds of all types, to reduce bacterial burden and help to accelerate wound healing times. Lavage can be carried out by the veterinary nurse and is required throughout management of a wound, and not only at first presentation.
KEY POINTS
- Lavage is one of the most important first-line methods of reducing infection in wounds.
- Solutions should be isotonic and wounds irrigated copiously to reduce bacterial burden.
- Debris, necrotic tissue, bacteria and dressing remnants can be removed by using lavage.
- Surfactants are becoming more widely used in reducing bacterial biofilms to help improve wound healing times.


