An unvaccinated, indoor-outdoor, 9-year-old male neutered Oriental cat presented with a 2 day history of lethargy, anorexia and vomiting. On examination the normally bright and vocal cat was dull and unresponsive. Body condition score was normal at 6/9. Mucous membranes were tacky, and oral lesions were observed in addition to halitosis. On auscultation the patient was bradycardic with a heart rate of 128 beats per minute (bmp) (normal range 110–220, Jack and Watson, 2008), but pulses were matching. Systolic blood pressure was recorded initially by indirect Doppler measurement (CAT Doppler, Thanes Medical) and found to be 130 mmHg (reference range: 120–170 mmHg, Jack and Watson, 2008). On abdominal palpation the anterior abdomen was tender and his bladder empty. Rectal temperature was slightly low at 37.0°C (normal range 38.0–39.0, Jack and Watson, 2008). Blood tests were collected for full haemotology and biochemistry with electrolytes and a 24G intravenous (IV) canula was placed in the cephalic vein immediately. IV fluid therapy (Hartmanns, Aqupharm, animalcare) was started at twice maintenance. In-house blood results indicated severe hyperkalaemia and azotaemia (Table 1), as well as a stress leucogram (neutrophilia with lymphopenia); IV fluid therapy was changed to 0.9% saline. The owner knew of no possible toxin exposure and did not report any other significant events such as trauma or recent illness. The patient had last been seen at the hospital 2 years previously for pre-anaesthetic blood tests (all normal) and dental treatment including extractions. The blood results along with clinical signs and history suggested acute kidney injury (AKI), although other possible differential diagnoses included acute decompensation of pre-existing kidney disease (acute-on-chronic), ruptured bladder or (very rarely in cats) hypoadrenocorticism (DiBartola, 2006). To rule out Addison's disease an adrenocorticotropic hormone (ACTH) test was performed once the patient was stable; the results subsequently came back normal.
| Blood parameter | On admission | 19 hours after treatment started | Normal range |
|---|---|---|---|
| K+ (mmol/l) | 7.3 | 7.8 | 3.5–5.8 mmol/l |
| UREA | 74.7 | 107.2 | 5.7–12.9 mmol/l |
| CREA | 1 955 | 3 072 | 71–212 µmol/l |
| PHOS | >5.19 | 5.96 | 1.0–2.42 mmol/l |
Biopsies may aid in determining the severity of the injury, but are rarely used (Monaghan et al, 2012). In this case, the patient was too dehydrated to consider sedation or anaesthesia for biopsy retrieval. Any hypovolaemic state must be corrected to prior to sedation or anaesthesia to prevent further damage to the kidneys that may result from hypotension and poor kidney perfusion (Pittari et al, 2009).
AKI
AKI is defined as a rapid decline in renal function (Jack and Watson, 2008). It is differentiated from chronic renal disease (CRD) by their different pathologies. In AKI, blood does not adequately perfuse the renal cortex, which affects ion excretion by the otherwise healthy nephrons; however, in CRD some of the nephrons are permanently diseased. For this reason with AKI much of the damage can be reversed if treated aggressively enough to prevent the formation of permanent lesions (Michell et al, 1989). In AKI, and severe CRD, animals usually present with severe depression, vomiting, anorexia and polydipsia. The two conditions may be differentiated by considering the patient's history, serum biochemistry, urine production and by examining tissue biopsies. The easiest and cheapest of these is to monitor urine production; although AKI has both oliguric and polyuric forms, CRD is always associated with polyuria until the animal decompensates completely and becomes anuric (see Table 2 for AKI/CRD comparison). In the oliguric form of AKI, little urine is produced even when dehydration is corrected with fluid therapy. AKI typically presents in the oliguric form, but milder forms and some specific types of nephrotoxins can produce the polyuric form. To complicate matters, after an animal begins to recover from AKI, a period of polyuria may be observed for 24–72 hours while excess solutes or accumulated fluids are expelled (Schaer, 2003). For this reason it is especially important to monitor and record urine output and weight often and carefully while hospitalised.
| Acute renal injury | Chronic renal disease | |
|---|---|---|
| Presentation | Depression | Depression |
| Vomiting | Vomiting | |
| Anorexia | Reduced appetite | |
| Oliguria | Polyuria | |
| Seizures* | Seizures* | |
| Muscle twitches* | Head bobbing* | |
| Examination findings | Dehydrated | Dehydrated |
| Uraemic breath | Uraemic breath | |
| Hypothermia | Hypothermia | |
| Large, sore kidneys | Abnormal kidney shape | |
| Oral lesions | Oral lesions | |
| Rubber jaw* | ||
| Biochemistry | Elevated urea, creatinine and phosphorus | As in ARI |
| Electrolytes | Hyperkalaemia | Hypokalaemia |
| Metabolic acidosis | Metabolic acidosis | |
| Haemotology | Lymphopenia | Lymphopenia |
| Normal PCV | Anaemia | |
| Urinalysis | Active sediment | Little sediment |
| Low SG | Low SG |
Understanding hyperkalaemia
Potassium is the major intracellular ion, which means that 95% of the body's potassium is inside the cells and only 5% outside in the serum (DiBartola, 2006). The movement of potassium and other ions in and out of cells is constant, but in a healthy animal stays within a very narrow range of concentrations. In the cat, serum potassium should be between 4.0 and 5.8 mEq/l (for potassium 1 mEq = 1 mmol) (Jack and Watson, 2008). The kidneys are the primary potassium balance regulator. Ordinarily, the serum concentration of potassium increases when an animal eats and cells take up potassium. Renal excretion of potassium then follows to restore the balance. In CRD this normal balance is kept in check because the remnant nephrons excrete more potassium to make up the difference and faecal excretion is also increased. However, in AKI there is no renal adaptation by the nephrons and because blood is not correctly travelling to the kidney cortex, the osmotic balance is disrupted (DiBartola, 2006). Ordinarily the kidneys retain sodium ions and excrete potassium and hydrogen ions but this system stops working, causing the serum potassium to increase. Furthermore, even when an animal is hyperkalaemic, if too many hydrogen ions are present (acidosis) they may bump even more potassium out of the cell, worsening the hyperkalaemia. If left untreated, hyperkalaemia can cause ventricular arrhythmias which may manifest as isolated ventricular premature complexes (VPCs), or worse. VPCs are depolarisations of the heart tissue that go from cell to cell instead of through the conduction tissue (Fuentes and Swift, 1998). In the most serious cases VPCs can progress to ventricular fibrillation, causing cardiac arrest and death. The patient was at serious risk of ventricular arrhythmias and for this reason was put on constant ECG monitoring with sticky electrodes secured with adhesive tape. Normal QRS complexes were observed.
Treatment plan to normalise blood values
Once it had been established that the patient's kidney injury was acute the primary goal was to correct his fluid deficit and hyperkalaemia. As Hartmann's solution contains potassium, intravenous (IV) fluids were exchanged for 0.9% saline (Aqupharm, animalcare). However, this course of action alone is not sufficient to allow the kidneys to expel the extra potassium (Michell et al, 1989). Administration of glucose assists in the reduction of serum potassium by stimulating the release of endogenous insulin, which then moves potassium into the cells. To achieve this, dextrose (Hameln pharmaceuticals) plus normal insulin (Hypurin Bovine neutral insulin, CP Pharamceuticals) was added to the saline and delivered intravenously (see Table 3 for dose rates). In healthy animals insulin need not always be added and the nurse should be sure to watch for signs of hypoglycaemia or monitor blood glucose (Bistner et al, 2000). The patient's blood glucose was intermittently monitored by ear prick using a handheld glucometer (AlphaTRAK, Abbott Laboratories) and found to be within the normal range. This protocol starts to have an effect within 1 hour. Sodium bicarbonate may also be used to move potassium into cells by increasing blood pH, but blood gas monitoring is needed to determine the correct dosage. To protect from the cardiotoxic effects of hyperkalaemia, 10% calcium gluconate treatment was also started (Table 3). Care must be taken to administer intravenous calcium gluconate slowly (over 10–20 minutes). Calcium gluconate does not affect the serum potassium in any way but restores normal membrane excitability so the potassium does not exert an effect on the heart tissue (DiBartola, 2006).
| Treatment goal | Medication and dose rate |
|---|---|
| Correct hyperkalaemia/acidosis | 1.5 g/kg of 50% dextrose plus 1U normal insulin per 3 g dextrose |
| Protect heart tissue | 10% calcium gluconate at 0.5–1.0 ml/kg intravenously (IV) slow, over 10–20 minutes |
| Protect heart tissue | Furosemide at 2.2 mg/kg IV |
Nursing goal: monitor fluid balance
In cases of AKI, it is especially important to monitor and record the animal's fluid balance, or ‘ins and outs’. In a euvolaemic animal, these ‘in and outs’ should match, and this can be estimated by weighing the patient throughout the day as daily weight fluctuations are reflective of fluid balance (Mazzaferro, 2013). Ins are the sum of the fluids given and consumed, including food and water. Outs are sensible losses, such as urine production and vomit, and insensible losses such as fever or metabolism. Mazzafero suggests 20 ml/kg/day as an estimate of insensible losses (2013). Although the patient's dehydration was improving (he gained 300 g overnight), he had still not urinated and did not have a significantly sized bladder. Osmotic and loop diuretics can be administered to help restore urine output once hydration is achieved and can help to manage electrolytes and hydration in this respect. Common choices are furosemide and mannitol, although dopamine is no longer recommended due to possible negative effects (Monaghan et al, 2012). It was decided after 21 hours of fluid therapy, with little to no improvement in hyperkalaemia or significant urine production, to begin furosemide intravenously. This can be repeated after 30 minutes if diuresis is not induced (Schaer, 2003). This treatment effected the most significant improvement in the patient's condition and blood parameters (see Figure 1 and Table 1).
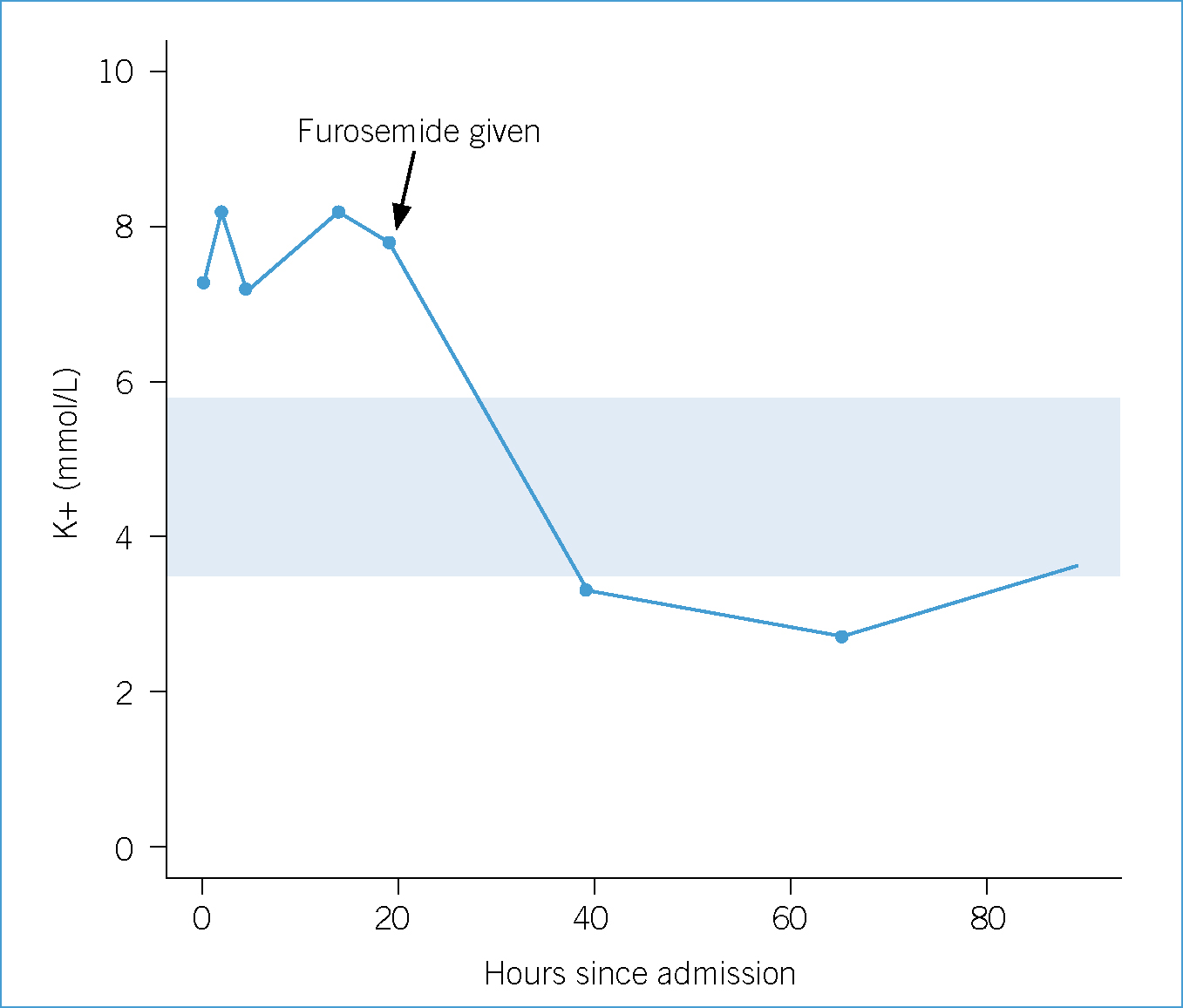
An intensive care hospitalisation sheet is imperative for clear recording of these parameters. This allows nurses to communicate with other nurses and veterinary surgeons the amount of water consumed and approximate volume of urine passed on the patient's hospital record. To approximate the volume of urine passed the patient's bedding was weighed (and 1 g taken to be equivalent to 1 ml); a more accurate way would have been to place a urinary catheter with a closed-system collection set. Urine production should net approximately 1–2 ml/kg/day. However, placement of a urinary catheter would have required sedation and initially the patient was too dehydrated to consider sedation.
Treating the patient's hyperkalaemia, dehydration and oliguria with fluids and diuretics also addressed his azotaemia (Table 1). Two days after his initial presentation the patient's potassium was in the normal range and he was bright and eating voraciously. In situations where this treatment does not work, it is possible to initiate specialised treatments that would require referral to a centre equipped to offer cation exchange resin, peritoneal dialysis or intermittent (traditional) haemodialysis (DiBartola, 2006). Continuous renal replacement therapy (CRRT), a gradual form of dialysis that takes place over days rather than hours, has recently become available in Britain and at the time of writing is only available at the Royal Veterinary College near London (Cooper, 2013). CRRT is useful in cases of renal failure that do not resolve with aggressive medical management as described in this article. Diehl et al (2008) reported that 44% of cats were discharged from hospital after treatment with CRRT, although complications may include iatrogenic hypokalaemia and acidosis, calcium disturbances, and anaemia among others. For a patient with AKI, ideally the acid–base status should be monitored, which requires specialised equipment. In the patient's case, having accurate electrolyte and biochemistry values was of the utmost importance in order to evaluate the treatment. This meant preparing dilutions of the patient's serum for the VetTest Chemistry Analyzer (IDEXX) to record the true biochemistry parameters so that his progress could be monitored accurately (Figure 2). Due to the importance of frequent blood sampling it was essential not to bruise the blood collection sites, and to reduce the number of needle sticks, medications were given intravenously (IV) where possible.
Improving perfusion and correcting acidosis can quickly turn hyperkalaemia into hypokalaemia (Schaer, 2003). This occurred in this case because polyuria is frequently observed during recovery from AKI and the patient's fluid rate was not decreased after the improvement of the blood parameters, meaning excess fluids were excreted along with potassium. This resulted in his serum potassium dropping to 2.7 mmol/l, which required supplementation. When supplementing potassium intravenously it is important to make sure that the rate of potassium delivery does not exceed 0.5 mEq/kg/hour IV. In retrospect, once the patient's potassium was in the normal range and his dehydration corrected the daily fluid volume could have been reduced accordingly with respect to his urine output but clinically he ate more and was brighter when maintained on a higher infusion rate. Although no clinical signs of overload were observed, such as peripheral oedema or respiratory distress associated with pulmonary oedema, a reduction of his fluid rate would have prevented the hypokalaemia that ensued. However, every effort to reduce the patient's fluid rate resulted in a return of the dehydration, followed by depression and a reduced appetite.
Treatment plan to get the patient home
Animals suffering with renal failure frequently have oral ulcers and gastric irritation, which exacerbates their inappetance. To treat the patient's nausea and ulcers he was medicated with the acid blocker ranitidine IV (Zantac, GlaxoSmithKline) and mucosal protectant sucralfate (Antepsin, Chugai Pharma UK) orally. Additionally, the patient was treated with amoxicillin/clavulanate (Synulox, Zoetis) by subcutaneous injection while hospitalised since the cause of his AKI was unknown and infection could not yet be ruled out. The patient continued to recover and by Day 4 all his blood parameters, both on biochemistry and haemotology, were within acceptable levels. A recheck 10 days later revealed he continued to thrive at home and blood parameters were all normal, although his urine specific gravity was only 1.015, suggesting possible long-term renal tubular damage (Figure 3).
Approximately 3 weeks after his initial hospitalisation the patient quickly deteriorated and was rehospitalised. On serum biochemistry his kidney values were increased again with hyperkalaemia. It was considered that the patient may have suffered his first bout of AKI due to an unknown toxin, and that he had now been exposed to a second time. However, as toxicity was unlikely based on the history, an underlying pyelonephritis was also considered given that his relapse occurred after finishing antibiotic therapy. Only 7 days of antibiotics were prescribed, which may have been insufficient for the treatment of a pyelonephritis. The same rigorous treatment protocol was begun and this time his recovery took longer. Two weeks after being admitted for a second time, the patient was doing well and was discharged on a renal diet (k/d, Hill's Prescription Diet), 6 weeks of oral marbofloxacin (Marbocyl P, Vétoquinol) and famotidine (Famotidine, TEVA UK) in accordance with a presumptive pyelonephritis diagnosis. Two months after his initial presentation the patient's weight was stable and his hydration was good; however, his urea and creatinine were both slightly elevated and his urine specific gravity 1.018. For several months the patient's owner reported that even with AKI he was bright and playful at home, chasing bugs and other household pets. However, 8 months later he suffered an acute decompensation, and although treatment was started the patient did not respond. The patient was humanely euthanised with his owner present.
Nursing goal: encourage and support natural feline behaviours
The patient's multiple episodes of AKI meant that he spent many days hospitalised, which was difficult for both him and his family. Ordinarily an indoor-outdoor cat, the patient was very social, accustomed to lots of attention at home and lazy hours in the garden. His illness and confinement had a significant impact on his grooming habits in particular. The veterinary nurses took time daily to groom his face and perineum with damp cotton wool and apply a soothing lotion (arnica cream (Arnicare, Nelsons)) to any bruised venipuncture sites. The veterinary staff made sure to set aside time for tender loving care (Figure 4) and also encouraged his owner to visit. The patient's family were made to feel welcome to spend time with him and were encouraged to bring his own food and blankets from home. The Feline-Friendly Nursing Guidelines published by AAFP/ISFM are very helpful in providing comprehensive instructions on making a cat's stay in hospital as stress free as possible (Carney et al, 2012) and the methods we implemented for this patient are summarised in Table 4.

| Problem or concern | What you can do |
|---|---|
| Monitoring ins and outs |
|
| Poor appetite |
|
| Cleanliness |
|
| Physical comfort |
|
| Mental comfort |
|
Conclusion
Early diagnosis of AKI and aggressive treatment can result in remission, but mortality rates are reported as high as 64% (Lee et al, 2012). However, excellent nursing care can make patients more comfortable and keen observations will help to monitor treatment and aid veterinary surgeons in tailoring treatment protocols. For those that do survive, approximately 50% will suffer from some degree of chronic disease that can be managed at home and by frequent veterinary monitoring (Monaghan, 2012).

