Historically, small mammals have been viewed as high-risk patients under anaesthesia. Small patient size and associated difficulties with this, preparation of equipment and the patient, monitoring techniques and recovery, are covered in depth in this article, in the hope of providing a comprehensive overview of the perioperative role of the veterinary nurse when performing anaesthesia in small mammals. This article is part one of a series of articles designed to provide an outline of the principles of anaesthesia in exotics and instil confidence in the veterinary nurse to be an advocate for their exotic animal patients.
Pre-anaesthetic preparation
Equipment
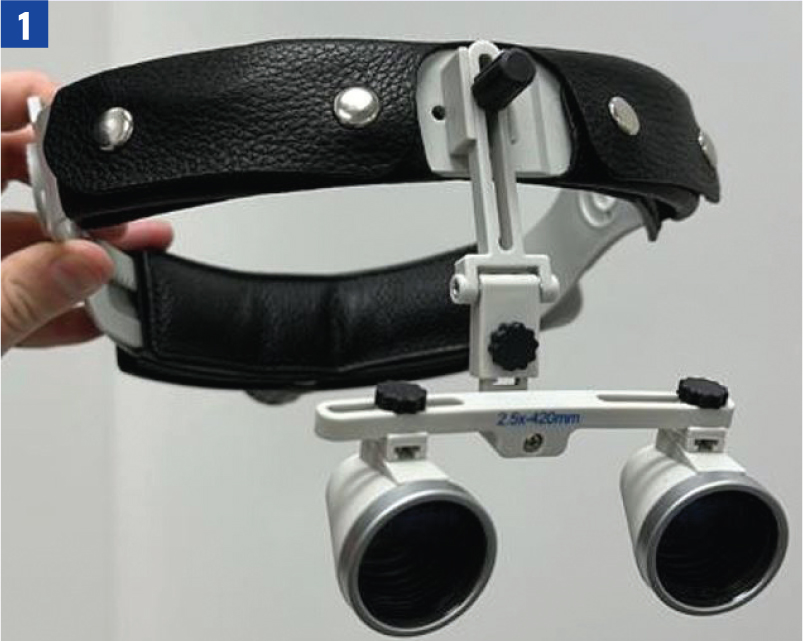
All equipment should be pre-prepared for the procedure in question, in order to maintain focus on patient monitoring once anaesthesia has begun. For very small mammalian species, surgical loupes – a magnifying head piece – can be useful to better visualise the surgical site and enable the veterinary surgeon to carry out procedures on a much smaller scale, with greater precision (Figure 1).
Environmental factors
A large surface area to volume ratio in many exotic patients means that cutaneous heat loss and evaporation of body fluids occurs much quicker than in dogs and cats (Scarabelli and Nardini, 2019). This can be minimised in several ways: prewarming fluids, either through use of a fluid warming cabinet, baby bottle warmer or simply microwaving the fluids. If using a microwave, care must be taken to heat in small bursts and mix well to dissipate the heat and avoid hot spots within the bag.
Scrub solution should also be pre-warmed; pre-diluted chlorhexidine or povidone-iodine can be drawn into a 20 ml syringe and placed in a baby bottle warmer until needed (Figure 2).

The surgical suite should be heated to the appropriate temperature for the species undergoing anaesthesia. Research suggests that an environmental temperature of 28°C is optimum for preventing hypothermia post-operatively in rabbits (Edis et al, 2022). Due to limited research in other species, it would be sensible to extrapolate this information for use in other small mammals. Some small mammals, such as chinchillas, are particularly susceptible to overheating due to their specialised coat, therefore core temperature should be monitored closely throughout the procedure and into recovery. Small rodents will lose heat rapidly and it may be difficult to restore normothermia once becoming hypothermic. Therefore, particular attention should be paid to not only the environmental temperatures of the theatre, but also heating devices provided to the patient in order to maintain an appropriate core temperature. Rufiange et al (2020) found a positive correlation between prewarming rodents with delaying the onset of hypothermia during general anaesthesia. Blankets or mats that require pressure to activate heat are not suitable for small mammals due to their small body size.
Patient evaluation
Many small mammal pets are prey species; stress caused by the sight and smell of predators can result in stimulation of the adrenergic system and the subsequent release of endogenous catecholamines. This leads to sensitisation of the myocardium and tachycardia, hypertension and reduced gut and kidney perfusion may be seen (Scarabelli and Nardini, 2019). Small mammals should be housed appropriately, away from predators (which includes ferrets) and loud noises, to reduce the effects of stress prior to anaesthesia.
The ASA score was produced for human use by the American Society of Anaesthesiologists, and adapted for use in animals. The framework acts to identify patients who are at greater risk under anaesthesia and helps to provide a basis for an anaesthetic protocol, based on the needs of the individual. The patient will receive a score of 1–5, with 5 being the most at risk.
Historically, this model has been used extensively in dogs and cats, but in recent years its use in exotic animals has also been highlighted. ASA grading has been described in rabbits, rodents and pigs, with results falling in line with those seen in dogs and cats; anaesthetic risk increases as the ASA status increases (Ishida et al, 2014; Portier and Ida, 2018; Bennett and Lewis, 2022).
Many small mammals are prone to respiratory diseases (Johnson, 2020). It may not be apparent while the animal is awake, but may be exacerbated by the respiratory depressant effects of anaesthesia gases and opioid drugs. This means most small mammals will automatically be classed as ASA II despite appearing healthy.
Fasting
Fasting animals prior to anaesthetic reduces the risk of vomiting and aspiration into the lungs, which can prove fatal. Herbivorous species, ie rabbits and most rodents, cannot vomit, therefore fasting prior to anaesthesia is not necessary. Fasting may also cause hypoglycaemia, due to their high metabolic rate, and gastrointestinal ileus in these species (Allweier, 2016). Some species, such as guinea pigs and hamsters, will hold food in their mouth so removing food from their enclosure 1 hour prior to anaesthesia, and then cleaning out the mouth with a cotton bud prior to induction is recommended (Glendinning, 2022).
Other small mammals such as hedgehogs, sugar gliders and ferrets can vomit and therefore require fasting. Due to increased metabolism of these species, a fasting time of 3–4 hours depending on the species, is generally recommended (Matchett et al, 2012; Graham and Knafo, 2021).
Catheterisation
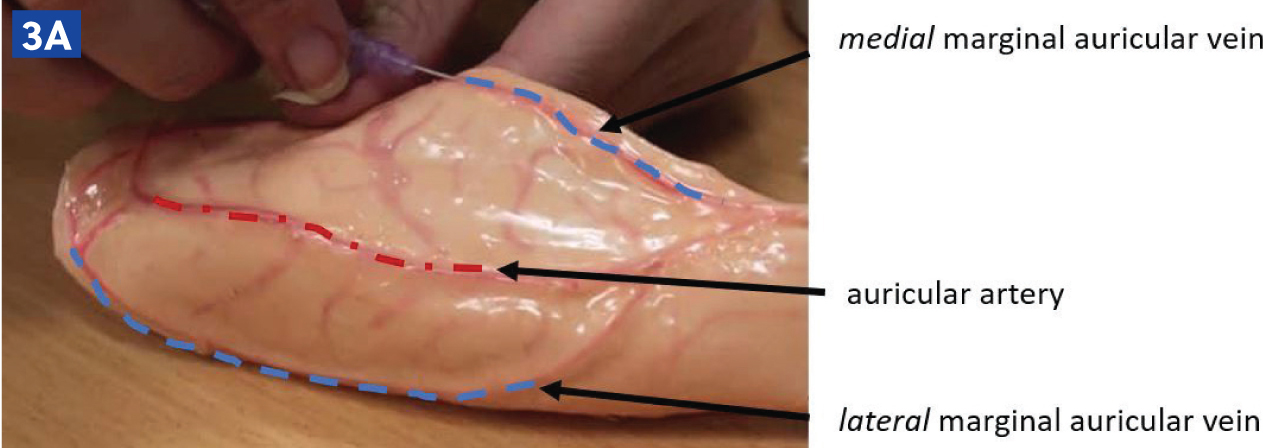
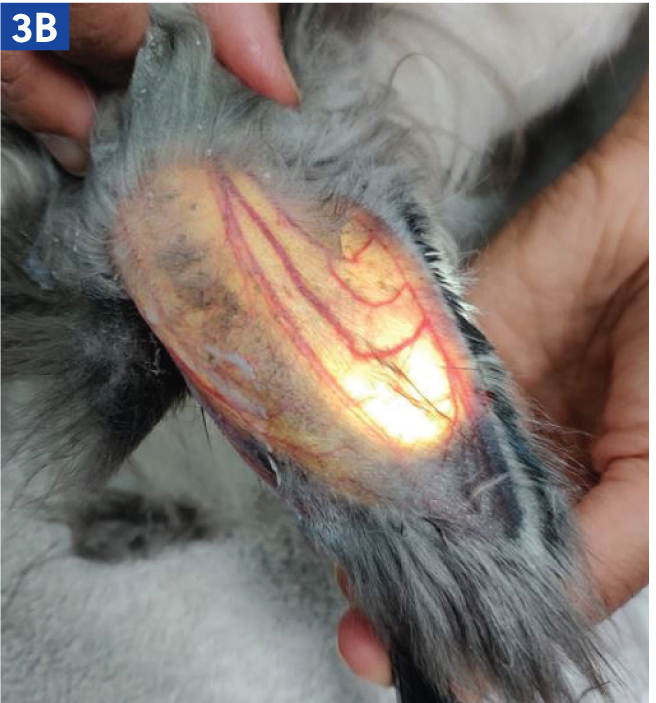
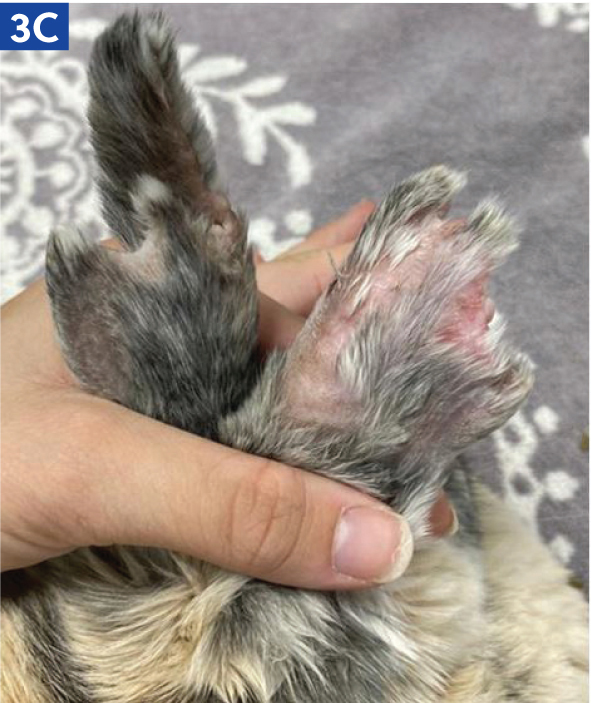
Rabbits may have an intravenous catheter placed in their marginal auricular vein than runs along the lateral and medial aspects of the pinna (Figure 3a and b). In small rabbit breeds, such as Netherland Dwarfs, the medial branch of the auricular vein may need to be used, as this is often larger. The auricular artery should be avoided, as blood clots in this vessel can lead to ear necrosis (Figure 3c). Catheters can also be placed in the cephalic vein, although conscious rabbits may resent the restraint required to extend the leg in order to place a catheter.
In larger small mammals, such as chinchillas, ferrets, guinea pigs and some hedgehogs, the cephalic and saphenous veins will accommodate a catheter; however, sedation or anaesthesia is often needed to facilitate this. In rats and other species with thick tails, the lateral tail vein can be catheterised with relative ease (Figure 4).
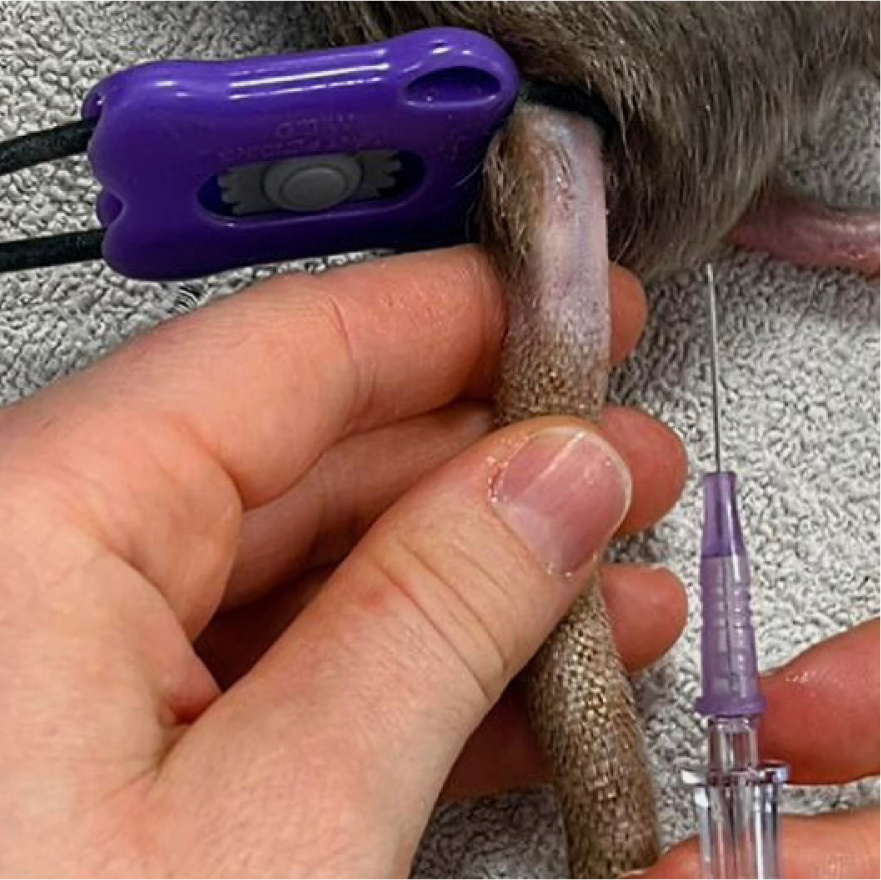
Some species have adapted to have particularly thick skin; these include but are not limited to guinea pigs, ferrets, European hedgehogs and some rats; and therefore, may require a cut down procedure in order to access the vein. The skin should be pulled laterally, and a small nick using a blade should be made over where the returning skin will sit on top of the vein; this will allow for easier placement of the catheter.
Skin preparation
In small mammals, minimal clipping is advised to avoid heat loss as much as possible. Care should be taken with species that are known to have thin skin such as rabbits, as clipper blades can easily cause cuts in the skin if they are not adequately maintained (Hollwarth and Vickery, 2020). As with dogs and cats, small mammal skin should be aseptically prepared with diluted chlorhexidine or povidoneiodine. A systematic review by Marchionatti et al (2022) in small animal veterinary surgery indicated no difference in the incidence of postoperative surgical site infections or skin bacterial colonisation when using either chlorhexidine or povidone–iodine for surgical skin preparation.
Final preparation with alcohol should be avoided in all exotic species, as it will cause further heat loss through evaporation (Marchionatti et al, 2022).
Induction of anaesthesia
Premedication
Premedication should be discussed as a clinical team, with the animal's signalment and history taken into account. Patients should always be weighed prior to giving any drugs, including fluid therapy. Not all procedures will require premedication, but those that are expected to be painful or invasive should be preceded by premedication as gaseous anaesthetic agents do not provide anti-nociception. Some premedication drugs such as midazolam, can also provide anxiolytic and amnesic effects, which can help to facilitate handling and minimise post-operative stress, as well as reduce the concentration of anaesthetic gas required (Van Zeeland and Schoemaker, 2021). Premedication drugs may be given subcutaneously, intravenously, intramuscularly or intranasally, depending on species, condition and stress levels.
A study by Askar et al (2020) investigated the bioavailability of buprenorphine given subcutaneously versus intramuscularly in rabbits. They found a lower bioavailability and lower maximum serum concentration, but a longer half-life when administered subcutaneously compared to intramuscularly; therefore, for immediate pain relief, it is recommended to administer analgesia intramuscularly or intravenously, but once pain levels are controlled, subcutaneous administration may be useful to sustain analgesic serum levels in rabbits (Askar et al, 2020). Additionally, Van Zeeland and Schoemaker (2021) identified a minimal delay in onset of anaesthesia (2 minutes) and markedly less patient stress, when using the subcutaneous route over the intramuscular route for premedication of ketamine-medetomidine combination in rabbits.
Induction
Inhalant anaesthesia is widely used in exotic anaesthesia, in conjunction with injectable agents. Discussion of anaesthetic gases will be covered later in this series.
Knock down boxes can be used for most small mammals, to induce anaesthesia. The box should allow space for the animal to sit comfortably without causing additional stress, but not be so large that it takes a long time to fill with anaesthetic gas and therefore, prolongs the induction time and increases patient stress levels.
One species that should never be induced via an induction chamber is the rabbit; this species will hold their breath indefinitely, which can lead to sudden death. These animals should be premedicated adequately to induce sedation, then induced to effect intravenously, where possible. This will enable intubation and ventilation in order to prevent apnoea and potential fatality.
Larger mammals, such as guinea pigs and ferrets, can be induced via face mask; however, the restraint needed can be stressful, and so the recommendation is to induce via intravenous agents where possible (Van Zeeland and Schoemaker, 2021).
Intubation
Intubation can be achieved in larger small mammals, either by using an endotracheal tube or the soft outer sheath of an intravenous catheter in smaller species. Intubation in rabbits requires practiced skill and specific positioning (Figure 5a and b). This can be achieved via otoscopic guided, endoscopic guided or blind technique; a local anaesthetic spray can be used to minimise laryngospasm.
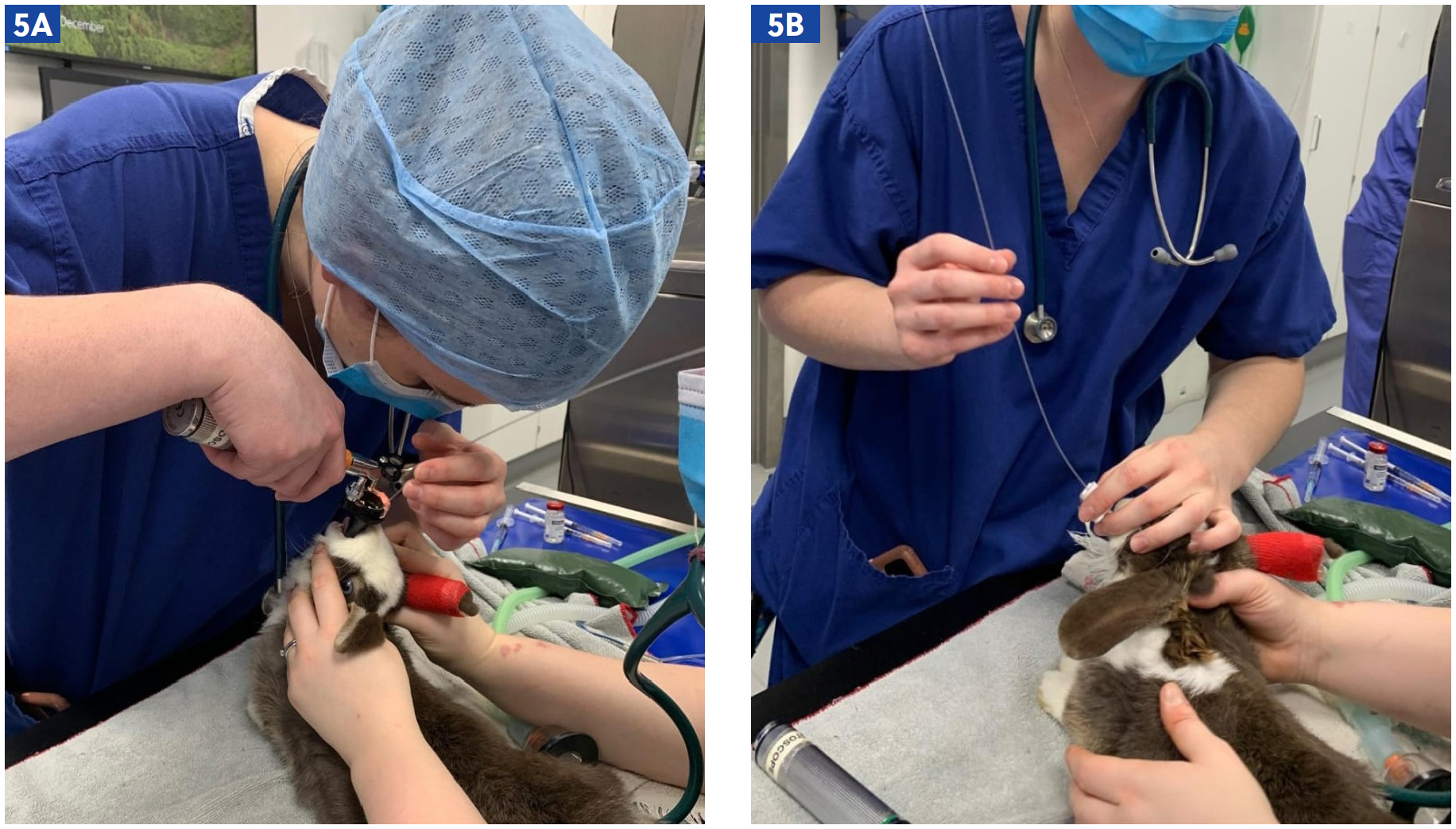
Grint et al (2006) reported on three cases of post-anaesthetic stricture in rabbits, following blind intubation without local anaesthetic spray. It could not be concluded whether traumatic or chemical injury was the cause, however it highlighted the necessity to replace endotrachael tubes frequently and to disinfect or rinse them well between patients. It also posed the question as to whether the lack of local anaesthetic spray correlated to the injury; additionally, it highlighted that intubation and movement of the patient after intubation should be completed with great care.
Although having a patent airway may seem advantageous, it is important to weigh up the likelihood of mucus obstruction occurring in a small tube and causing a full occlusion, versus maintaining the patient on a mask. Therefore, it may be inadvisable to intubate very small species such as rats.
In species that we cannot intubate, maintenance of anaesthesia can be via a tight-fitting mask, or laryngeal mask. A laryngeal mask should always be used in conjunction with a capnograph to ensure patency, as movement of the tube can cause fatal occlusion. In very small species, the end of the circuit can be used as a tight-fitting mask (Figure 6).

Maintenance of anaesthesia
Anaesthetic monitoring
Much of the anaesthetic monitoring equipment encountered will have been designed for dogs and cats, and therefore may not give a reliable reading in exotic patients. Probes may be too large and veterinary nurses may need to get creative with where these are placed. All measurements should be documented at least every 5 minutes, but due to the fast-paced nature of exotic animal anaesthesia, readings should be taken every couple of minutes. The first measurement should be taken at or directly after induction, and from then onward trends should be monitored. Given the wide variety of species that fall under exotic animals, it is far better to monitor the trend of an individual's parameters than follow textbook values; deviation of values from the trend can highlight changes in the physiological state of the animal under anaesthesia.
Anaesthetic depth can be measured using the pedal, palpebral and corneal reflexes, and anal or jaw tone. However, as prey species do not react the same way as dog and cats do, there are certain small mammal-specific adaptations to be aware of.
Rabbits will only blink every 5–6 minutes, so the palpebral reflex should not be relied on as the sole indicator for anaesthetic depth (Foote, 2020). Tonic immobility is considered an anti-predator behaviour, whereby prey species become apparently paralysed and unresponsive to external stimuli; it often occurs in response to an extreme threat, such as being captured by a perceived predator. It differs from freezing behaviour as it occurs after the predator has detected or made contact with the prey, whereas freezing occurs before detection, and acts as a form of camouflage. Tonic immobility also causes a release of catecholamines which can alter menace and pupillary light reflexes (Carli and Farabollini, 2022). To lessen the effects of tonic immobility, pre-medication and an appropriate length of induction via gaseous anaesthesia in sternal recumbency are usually effective.
When testing corneal reflexes in smaller patients, a damp cotton bud can be used to avoid injury. Rabbits will display a much firmer jaw tone under anaesthesia than a dog or cat due to large masticatory muscles and a small oral cavity, so it is important that the veterinary nurse familiarises themselves with this in order to avoid an anaesthetic that is too deep. Similarly, but often more pronounced than rabbits, ferrets have a very strong jaw tone, particularly on induction for intubation.
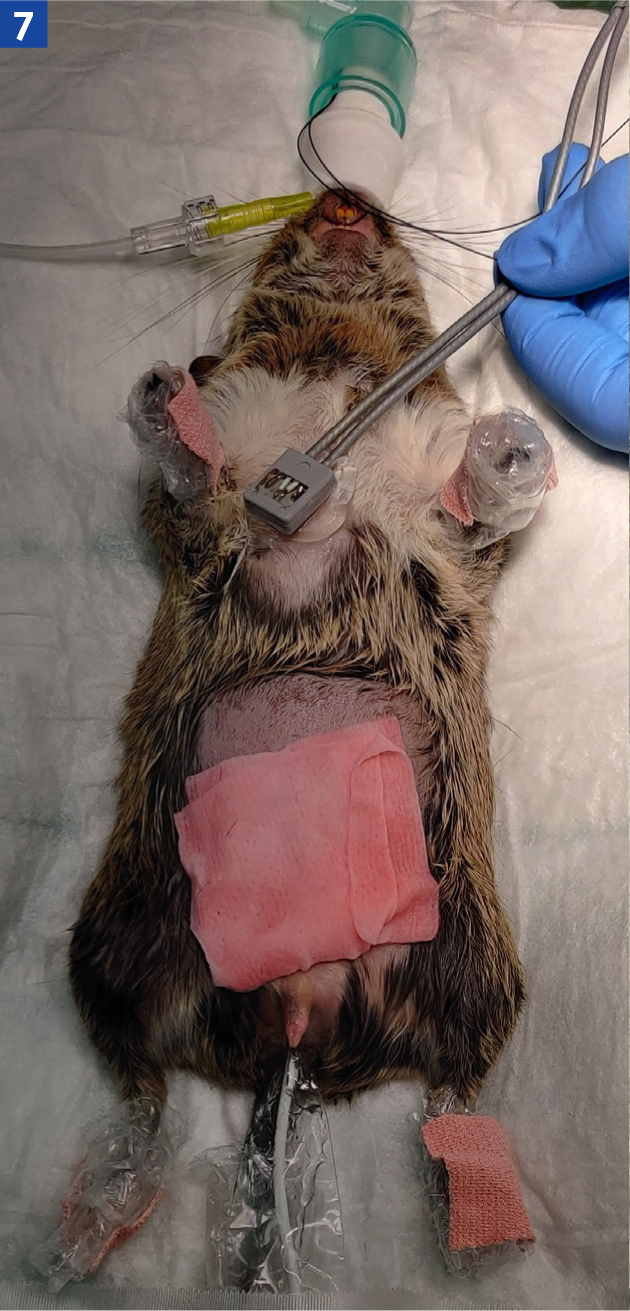
A doppler probe taped onto a tongue depressor and then placed over the heart (Figure 7) will provide an audible reading to the entire operating theatre, and the tongue depressor allows for changes in positioning throughout the procedure. The doppler can also be used over the femoral artery on the medial aspect of the thigh if the chest is not accessible. Evaluation of the femoral pulse should be carried out regularly to assess pulse strength and regularity, in relation to auscultation of the heart. Weak pulses may indicate hypotension, whereas excessively strong pulses may indicate hypertension; these findings should always be assessed alongside the rest of the patient's parameters (Ateca et al, 2018). The presence of arrythmias could be directly related to the anaesthesia, eg reduction in anaesthetic depth, or may be occurring due to a systemic disturbance, eg hypovolaemia, or concurrent disease (Hollwarth and Vickery, 2020).
Changes in respiration may be the first indicator of an anaesthetic complication. An increase in respiratory effort may indicate a blockage in the endotracheal tube or kinking of the tube. If face masks move and start to cause occlusion of the nares in obligate nasal breathing species such as rabbits and guinea pigs, increased respiratory effort leading to apnoea will be seen. Increases in respiration may also indicate nociception or a lightening of anaesthesia. Sidestream capnography alongside low dead space adapters are very useful in small mammal anaesthesia, as they help to minimise dead space and provide a visual trace to identify changes quickly. A blockage will show a prolonged expiratory phase on the capnograph (Ateca et al, 2018). A decrease in effort or rate may indicate a deepening of anaesthesia, and should be assessed against other vital parameters.
Hypotension can be a catastrophic effect of anaesthesia in exotic patients, especially those very small species. In addition to the underlying respiratory disease previously mentioned, chronic kidney disease is another commonly encountered problem, often in rabbits. As with any animal showing signs of chronic kidney disease, care should be taken to maintain normotension under anaesthesia to sustain perfusion to the kidneys (Scarabelli and Nardini, 2019). Gaseous anaesthesia alongside drugs such as opioids will cause hypotension, therefore these animals must be supported with fluid therapy throughout anaesthesia.
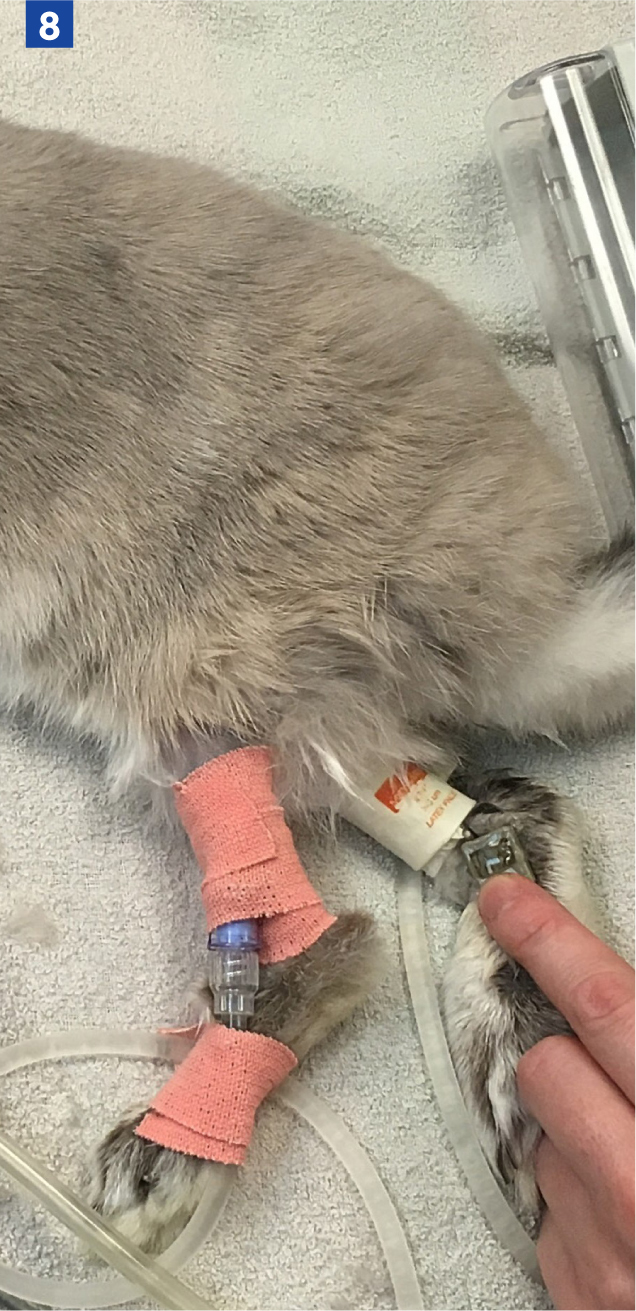
When assessing blood pressure in rabbits, avoid clipping the hair on the feet and hocks as this will predispose the patient to pododermatitis (Mancinelli et al, 2014; Graham and Knafo, 2021). Blood pressure in small mammals can be measured using the hindlimb (femoral, medial saphenous or dorsal pedal arteries – Figure 8), forelimb (dorsal carpal branch of the radial artery) coccygeal or auricular arteries, depending on species, the procedure and positioning of the animal.
There are mixed views on the usefulness of pulse oximetry in exotics; the clips are designed for dogs and cats and can therefore cause compression of the tongue in small mammals, reducing blood flow and giving a false reading. Non-haired feet may work in some species but may not be reliable, and excessive clipping to find a hairless patch of skin may lead to hypothermia.
Maintaining warmth in the operating theatre is important and has been highlighted earlier in this article. Heat pads, vinyl gloves filled with warm water and bubble wrap can all be used to encourage heat retention.
Recovery
It is important to carry out temperature control measures into the recovery period, with particular care being taken in species that may overheat quickly, such as chinchillas, or those that may lose heat very quickly, such as small rodents. Incubators are commonly used until the animal is normothermic and mobilising independently. Once awake, it may be difficult to obtain a repeat temperature, and so judgement of demeanour and mobility are also important.
Oxygenation may be beneficial for many species to encourage blow off of anaesthetic gases and return of normal respiration, especially if the patient was being supported by ventilation throughout anaesthesia. Rabbits and rodents are obligate nasal breathers, therefore the nares should be monitored closely for signs of obstruction.
Due to the fast metabolic rate of small mammals, long periods of inappetence can lead to hypoglycaemia and gastrointestinal ileus, which can be fatal if left untreated. Feeding as soon as the animal is adequately awake and can swallow, and good analgesia, fluid therapy and prokinetics where necessary, can all contribute to a smooth recovery. Supportive syringe feeding can be implemented by the veterinary nurse; a suggested volume is 10 ml/kg syringe feeds every 4 hours until the patient is eating well independently. Oxbow critical care formula, recovery diet or EmerAid are all suitable for syringe feeding when mixed with water. Ferrets and hedgehogs may lap EmerAid carnivore directly from a bowl; this should be offered quickly after recovery to avoid hypoglycaemia. In the author's experience, ferrets will often fall into very deep, unrousable sleep following recovery and eating; an important phenomenon for veterinary nurses to be aware of to avoid panic.
A good analgesic protocol will help with a smooth recovery and a faster return to normal food intake. Pain scoring using validated tools such as the rabbit grimace scale, the Bristol rabbit pain scale and the rodent grimace scale, can help to reduce subjectivity in scoring.
It may be necessary to continue fluid therapy into the postoperative period, depending on demeanour, vital signs including pulse quality and hydration status; the need for fluids should always be discussed with the veterinary surgeon.
Species-specific considerations
There are many species-specific small mammal quirks to be aware of, but a few that may present in general practice are:
- Hamsters and guinea pigs may store food in their cheek pouches or have food present in their mouths at all times, therefore their mouths should be checked and cleaned out prior to anaesthesia to reduce the risk of aspiration
- Elevate the chest of small mammals under anaesthesia to maximise lung volume, but pay particular attention to rabbits and guinea pigs; a large volume of abdominal viscera in these species can cause the weight of the abdominal organs to press on the diaphragm and decrease the ventilation volume of the lungs
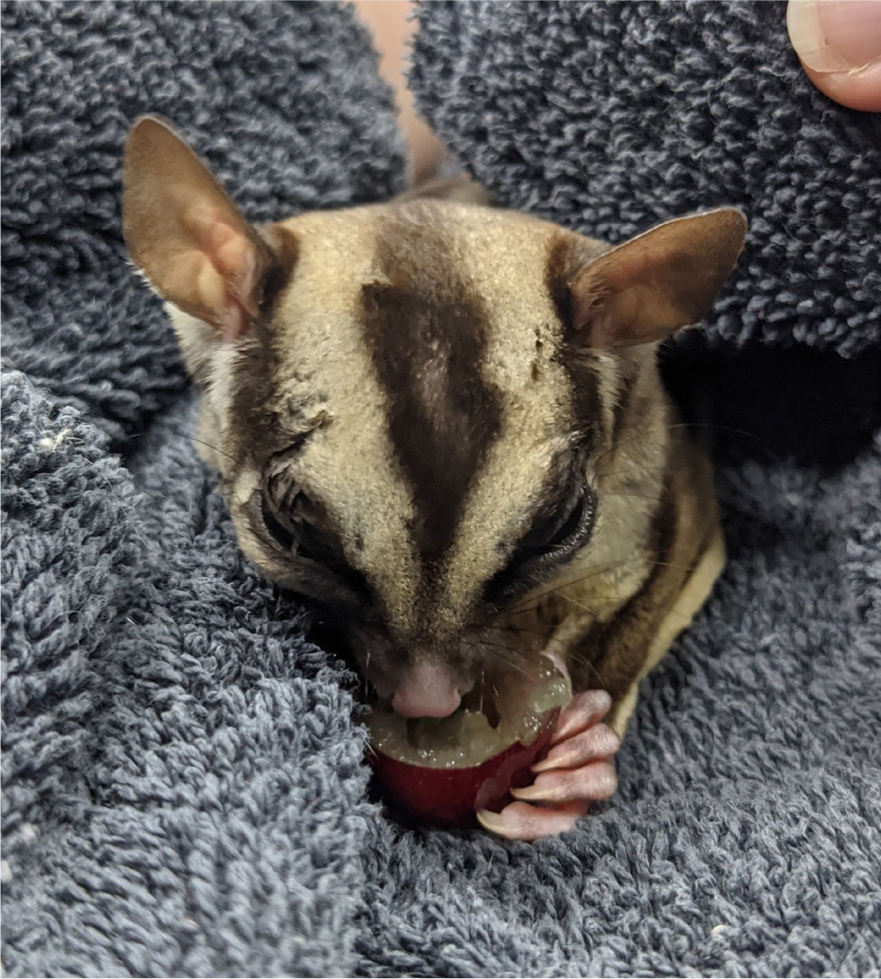
- It is not uncommon for sugar gliders and rats to self-traumatise postoperatively. Surgically preparing the skin with very dilute chlorhexidine or using sterile saline instead, and offering a sweet treat, such as a grape, on recovery to help distract the patient from their surgical site, are both ways to help reduce patient interference (Figure 9)
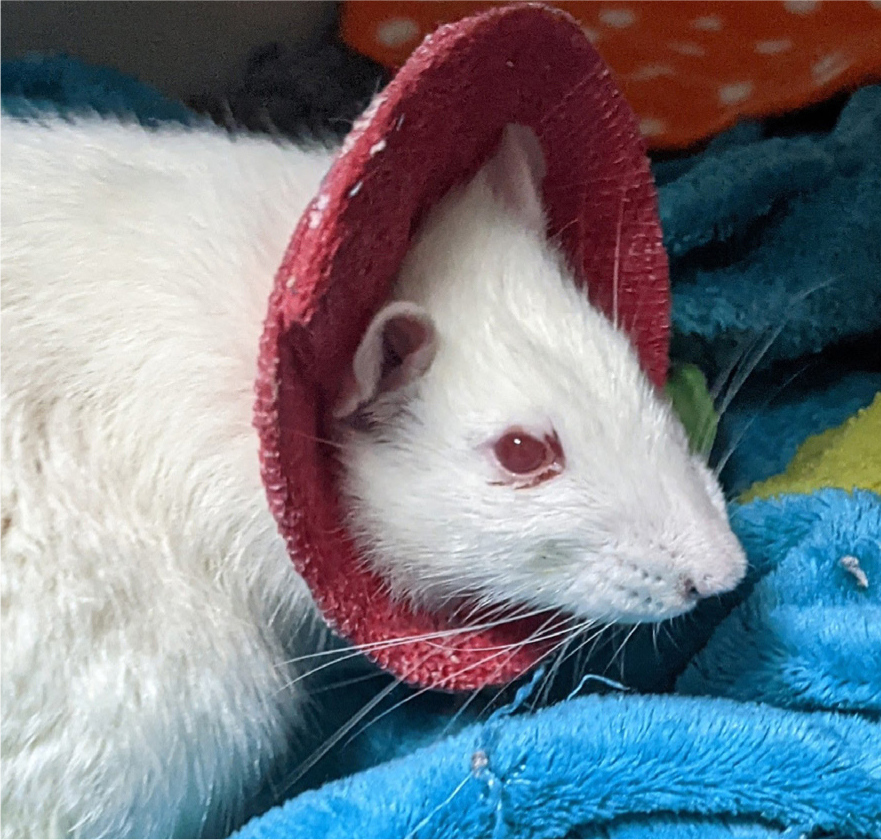
- Rats will sometimes need a collar or body wrap to prevent self-trauma post-operatively. When wearing a collar, the patient will be unable to groom normally therefore you may see porphyrin staining (a red-brown pigment) around the eyes, nose and on the fur (Figure 10). This is a normal secretion produced by the tear gland, which is normally groomed away. The patient may need help to groom
- Chinchillas have very dense fur; appropriately 60–90 hairs grow from each follicle (Prebble, 2011); meaning they can overheat very quickly. Particular attention should be paid to heating devices and core temperature checks of this species to avoid hyperthermia
- Many small mammals, such as rats and hamsters, have protruding eyes, and therefore are prone to corneal ulceration and other complications. Liberal ocular lubrication, before, during and after anaesthesia can help to reduce ocular complications. The eyes may also be gently taped shut during anaesthesia to avoid drying of the cornea; however, it must be ensured that the tape does not come into direct contact with the eye
- Ferrets may commonly present with adrenal pathology, insulinomas and various heart defects which may impact the anaesthetic; protocols should be discussed with the veterinary surgeon (Scarabelli and Nardini, 2019)
- Guinea pigs and gerbils are prone to hepatic lipidosis which should be a nursing consideration, especially if a period of appetence precedes the anaesthetic (Scarabelli and Nardini, 2019)
- Particular care must be taken to ensure small mammals are eating well after an anaesthetic. Hypoglycaemia and ileus are big risk factors in small mammals, in particular rabbits. Adrenergic stress and inadequate analgesia will reduce the mobility of the gastrointestinal tract and predispose the patient to developing ileus (Scarabelli and Nardini, 2019). A good analgesic protocol and pre-emptive prokinetic medications, can help to minimise these effects.
Emergency protocols
In small animal medicine, the recover guidelines were created to provide guidance to veterinary professionals for evidence-based management of cardiopulmonary cerebral resuscitation, basic life support and advanced life support, of dogs and cats (Fletcher et al, 2012).
The principles of cardiopulmonary cerebral resuscitation remain the same for small mammal species, but it is important to consider that they have a higher resting heart rate, which means compressions will need to be faster, around 120–140 bpm. Intubation, if not already completed, should be a priority to enable the provision of ventilation, and reversal of any drugs that can be reversed should take place. Core temperature should also be monitored closely as a significant rise or fall in temperature may occur during a crash scenario, and this can have a detrimental effect on a small mammal.
Emergency drugs
When preparing for procedures that carry a higher ASA status and therefore a higher risk of anaesthetic death, it is useful to pre-calculate and prepare emergency drugs for use if needed. Additionally, many exotic animals will weigh under 1 kg, and so these drugs may need diluting to achieve accurate administration. This process takes time and may not be achievable during an emergency arrest.
The easiest way to achieve this is by diluting the drug 1:10 with sterile saline or water for injection; see the below calculation for an example.
Patient: ‘Lucky’, 2-year-old, Fancy rat, ASA status 3–4
Weight: 400 g
Example dilution:
Adrenaline 0.003 mg/kg intravenously, conc. 1 mg/ml (Hedley, 2023)
0.4 kg x 0.003 mg/kg = 0.0012 mg / 1 mg/ml = 0.0012 mls
This tiny amount would be impossible to draw up accurately. To make this easier, the drug can be pre-diluted to make the volume being drawn up larger.
- Draw up 0.1 ml of the drug (adrenaline) and dilute with 0.9 ml of saline. This creates a 1:10 dilution, making this solution 0.1 mg/ml
- Divide the calculated volume (0.0012 ml) by the concentration of the diluted solution (0.1 mg), to get your new volume required (0.012 ml)
- The new volume will be 10 times greater than the calculated dose, due to the 1:10 dilution, and will therefore be much easier to draw up accurately. The new volume would be 0.012 ml, which can be drawn up in an insulin syringe.
Conclusions
Anaesthesia in small mammals can be challenging but ultimately rewarding. Ensuring that everything is prepared methodically can avoid stress during the procedure and allow for more focused and diligent monitoring of the patient. Intubation may feel like gold standard but may not always be the most appropriate choice, especially in small species where mucus occlusion of the tube is a real possibility. Never underestimate the importance of warmth, nutrition and a good analgesic protocol, and remember to be aware of species-specific quirks. Pre-dilution of emergency drugs can provide better accuracy and reduce panic during an emergency situation, and can be applied to all exotic species.
KEY POINTS
- Preparation is key to a smooth and well-planned anaesthetic; this should be a whole team effort.
- Changes in parameters in exotic species are often rapid, so diligent monitoring of vital signs and anaesthetic depth is key.
- A large surface area to volume ratio in many small mammals means that heat loss can be vast and rapid; awareness of perioperative thermoregulatory support is important.
- Monitoring trends in values, rather than focusing on what might be ‘normal’ for that species, is the key to noticing and remedying changes during an exotic animal anaesthetic.
- The veterinary nurse plays a pivotal role in the recovery period of the small mammal; ensuring fluid therapy, analgesia, warmth and nutrition are balanced to support a fast return to normal.


