The order Rodentia has more than 2000 species and these can be divided into the sub-orders Anomaluromorpha (springhares, rodents native to the semiarid steppes and dry savannas of Kenya and Tanzania as well as in southwestern Africa), Castorimorpha (beavers, pocket gophers, kangaroo rats and kangaroo mice), Hystricomorph or Caviomorpha (guinea pigs, chinchillas, degus), Myomorpha (mouse-like rodents to include mice, rats, hamsters and gerbils), and Sciuromorpha (squirrels and prairie dogs) (Bennett, 2022; Bennet and Lewis, 2022). This article will concentrate on anaesthesia in the smaller species seen commonly in general practice within the Myomorpha order such as mice, rats, hamsters, and gerbils.
Anaesthesia of these rodents is associated with a higher risk compared with anaesthesia of cats and dogs. This risk is a result of several anatomical and physiological characteristics in addition to the relatively short life span of these species (Richardson and Flecknell, 2009). Rodents are prone to hypothermia because of their high body surface area to volume ratio, the cooling effects of anaesthetic gases and reduced movement (Flecknell, 2009a). Rodents are also prone to hypoglycaemia because of their low glycogen reserves. A rodent's high metabolism means that drug metabolism and excretion are very fast, oxygen demands are very high, and central nervous system damage occurs within seconds of respiratory arrest (Hawkins and Pascoe, 2021a).
Pre-anaesthetic period
The pre-anaesthetic phase comprises a thorough pre-anaesthetic evaluation to include a clinical history and a physical examination, as well as selecting the appropriate pre-anaesthetic drugs and analgesia (Grubb et al, 2020; Gerrard, 2022).
Assessment of patient stability should be carried out and the rodent assigned to an American Society of Anesthesiologists (ASA) physical status classification from I to V (Table 1).
Table 1. American Society of Anesthesiologists physical status classification system score to act as a guide in addition to a physical examination of the patient
| Physical status | Criteria | Examples |
|---|---|---|
| ASA I | Healthy patient | Elective procedure, e.g. neuter |
| ASA II | Mild/localised systemic disease | Incisor burr |
| ASA III | Systemic disease without immediate risk to life | Gastrointestinal hypomotility |
| ASA IV | Systemic disease with immediate risk to survival | Foreign body |
| ASA V | Death within 24 hours without intervention | Severe trauma |
Prior to anaesthesia a health check should be completed; stress can cause a marked increase in respiratory and heart rates and this needs to be taken into consideration when transporting and handling these rodents. Like all prey species, rodents will hide signs of disease until the disease is too advanced for them to hide, and underlying subclinical pathologies may not be noticed. Rodents also have a short lifespan so could be classed as geriatric at 18 months to 2 years of age. The health check should be started while the animal is in its carrier (Figure 1). Assessing its respiratory rate before handling may give a more accurate measurement. Rodents are generally obligate nasal breathers, so it is imperative that they have clear nares, free of blood, pus and/or masses. Some rodents, especially mice and rats, will show porphyrin staining, which may be indicative of stress or ill health; this also can be noted before handling. Other signs of ill health that can be noted before handling the animal can include rough or unkempt coat, staining around the anal region, sunken eyes, and noticeable bony prominences. Any of these signs would warrant a discussion with the owner regarding further tests or treatment before anaesthesia is performed.

Once happy that the rodent looks visibly healthy it can be removed from its carrier to be examined fully in a quiet environment, away from any species perceived as predators such as cats, dogs, ferrets and birds of prey. Rodents can be difficult to handle and may bite if they are scared or not familiar with the person restraining them. Most rodents will allow handling by holding in cupped hands or around the chest in rats while supporting their bodyweight. Rodents must never be picked up by their tails without supporting the rest of their bodies. In the literature it states that they can all be carefully scruffed between thumb and forefinger ensuring that the skin is pulled in a cranial direction to prevent proptosis of their eyes. However, most hamsters, gerbils and rats actively object to this and the authors prefer to restrain the animal with a small towel instead. Heart rate is auscultated using a small stethoscope, rectal temperature can be taken if the animal is large enough, and mucous membrane colour should be noted (Table 2). It is uncommon for pre-anaesthesia blood sampling to be taken in small rodents, but it can be done, especially in larger rodents such as rats. For smaller rodents a brief general anaesthetic is required for blood sampling. A maximum amount of blood that is equal to 1% of the rodent's bodyweight can be safely taken (Lennox and Bauck, 2012).
Table 2. Vital parameters for Myomorph rodents
| Parameter | Mouse | Rat | Hamster | Gerbil |
|---|---|---|---|---|
| Bodyweight (g) | 25–40 | 300–500 | 85–150 | 85–150 |
| Temperature (°C) | 37.5 | 38 | 37.4 | 39 |
| Respiratory rate (per min) | 80–200 | 70–115 | 80–135 | 90 |
| Heart rate (per min) | 350–600310–840 | 250–350 | 250–500 | 260–300 |
It is vital that an accurate bodyweight is taken using scales that can measure small amounts from 0.1 g to be as accurate as possible when calculating the dosage of medication. Bodyweight is also a useful tool when monitoring treatment success or to detect the onset of disease.
Pre-anaesthetic fasting is unnecessary as rodents cannot vomit, but if deemed necessary should only be for a maximum of 45–90 minutes, any longer than 2 hours can cause postoperative hypoglycaemia. The exception to this would be if gastrointestinal tract surgery were to be carried out and a reduction in gut matter was beneficial. As rodents are coprophagic, if the stomach needed to be completely empty, droppings would have to be removed as they are passed. All rodents can have access to water until 1 hour before the anaesthetic. If the rodent is dehydrated, or has lost fluids through diarrhoea or blood loss, preoperative fluids can be given by the subcutaneous or intravenous route (Flecknell, 2009b).
Fluid therapy should be given before, during and after anaesthesia as rodents will dehydrate faster than a dog or cat even during a relatively short procedure, this is because of a rodent's large body surface area in relation to the volume (Girling, 2013). Intravenous fluids are always the best option but may not be possible in such small mammals. The loose skin over the middle body area can be utilised, and warmed fluids can be administered over several areas subcutaneously.
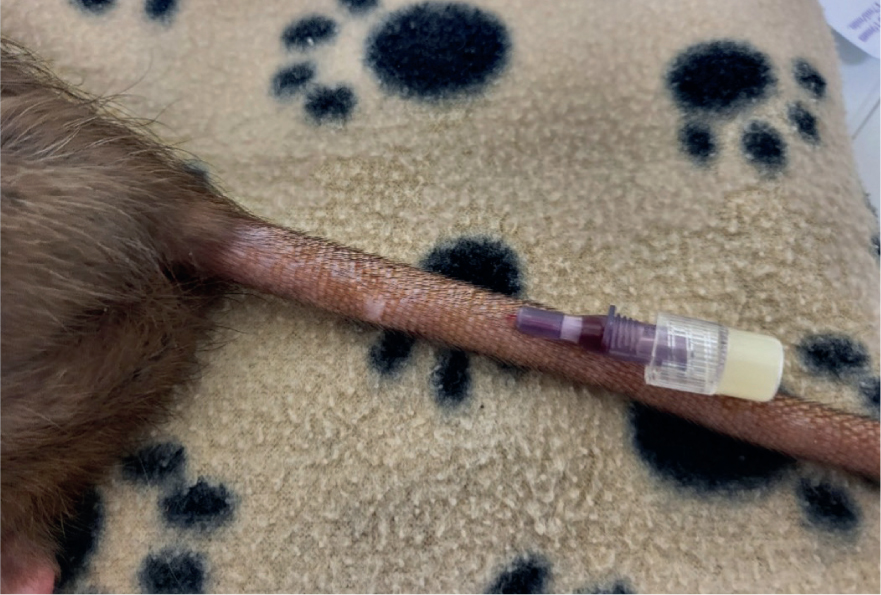
Vascular access can be achieved in the rat via catheterisation of the cephalic vein, the lateral saphenous vein, the femoral vein, or the superficial lateral tail veins (Figure 2). Once the cannula is in place, it is taped and bandaged. For additional security while the animal is under anaesthesia, a tongue depressor can be used to secure very small limbs (Hawkins and Pascoe, 2021).
Intraosseous (IO) catheterisation can be placed in the tibia via the tibial crest, or femur via the trochanteric fossa, in small rodents if vascular access is required for anaesthesia or during cardiovascular compromise. Hypodermic needles (18 to 25-gauge, I inch long) can be used depending on the size of the rodent. Strict aseptic technique is essential during placement and maintenance. The IO cannula should be flushed with heparinised saline twice daily if fluid is administered in boluses rather than continuously. Contraindications of IO cannulation include patients with a metabolic bone disorder or septic patients. Analgesia must be given because of discomfort of the limb and osteomyelitis must be considered as a potential adverse effect (Hawkins and Pascoe, 2021).
Pre-operative preparation of equipment and medications is crucial to improve patient safety under general anaesthesia and to minimise the time spent under anaesthesia (Gerrard, 2022). As with anaesthesia on any species, an anaesthetic checklist should be completed to include checking the anaesthetic machine, the breathing system and adjustable pressure-limiting valve, ensuring that there is adequate inhalant agent and oxygen available and to set up the monitoring and ancillary equipment.
Before any sedative or anaesthetic drug is given, the authors calculate emergency drugs and record them in a table on the anaesthetic chart. In a case of emergency, this ensures medication is administered rapidly. Emergency doses used are adrenaline at 0.01 mg/kg administered intravenously (IV) or IO, atropine 0.04 mg/kg IV or IO, flumazenil 0.01 mg/kg IV if reversing midazolam, and atipamezole to reverse medetomidine (given at an equal volume to medetomidine) (Gladden and Lennox, 2021).
Analgesia and pre-medication
Rodents are prone to self-mutilation after surgery; to minimise this multimodal analgesia is recommended (Table 3). As part of a multimodal approach the use of local anaesthesia should be considered as this will reduce the general anaesthetic requirement (Richardson and Flecknell, 2009). Lidocaine at 1 mg/kg, diluted 1:10 in saline solution is often prepared to infiltrate on the surgical site before incision. Bupivacaine at 1–2 mg/kg is also prepared as this is often administered via a splash block before closure.
Table 3. Examples of pre-medication and analgesic drugs in rodents
| Drug | Dose |
|---|---|
| Buprenorphine | 0.05 mg/kg subcutaneous (SC) every 8–12 h for rats0.1 mg/kg SC every 8–12 h for mice0.1 mg/kg SC every 6–8 h for hamsters and gerbils (Richardson and Flecknell, 2009) |
| Meloxicam | 1 mg/kg per os (PO)/SC every 12–24 h for rats (Richardson and Flecknell, 2009)0.5–1mg/kg every 24 h PO or SC for hamsters (Mayer, 2013)0.2–1mg/kg PO/SC every 24 h for gerbils. Based on doses for other rodents (Flecknell, 2001; Deutschland, 2011; Kubiak, 2021) |
| Atropine/glycopyrrolate | 0.04–0.4 mg/kg SC/intramuscularly (IM), can be given to prevent bradycardia and also to compensate for the excessive production of saliva and/or porphyrin secretions (Thompson and Bament, 2012) |
| Acepromazine | Can be used in rats, mice and hamsters at a dose of 0.5–1 mg/kg but must not be used in gerbils as it reduces the seizure threshold (Heard, 2004) |
| Diazepam | Can be used in rodents at a dose of 3 mg/kg but is oil based so can be painful when given intramuscularly |
| Midazolam | The authors prefer using midazolam at a dose of 1 mg/kg SC for myomorph rodents. Midazolam can be reversed with flumazenil on recovery if necessary |
| Hypnorm (fentanyl/fluanisone combination) | Can be used as a sedative at a dose of 0.04 ml/kg in rats and 0.01 ml/30 g in mice intramuscularly although Hypnorm is an irritant and can cause pain and discomfort at the site of injection. Hypnorm can be reversed using butorphanol 0.2 mg/kg or buprenorphine 0.05 mg/kg after the surgery and is licensed in the UK for use in rats and mice |
There are a number of pre-anaesthetic agents that can be used in rodents. The authors prefer pre-medication with buprenorphine and midazolam and find it is beneficial to minimise fluctuations in blood pressure and heart rate during induction of anaesthesia.
Medetomidine or dexmedetomidine can be used for sedation and analgesia but are needed in high doses which can cause bradycardia.
Morphine, buprenorphine and butorphanol can be given for analgesia which can reduce the amount of inhalant anaesthetic required but can also cause bradycardia.
Opioids can cause bradycardia when administered at higher dose rates, as part of an anaesthesia regimen, although using atropine can counteract these effects (Flecknell, 2009c).
Induction and anaesthetic maintenance
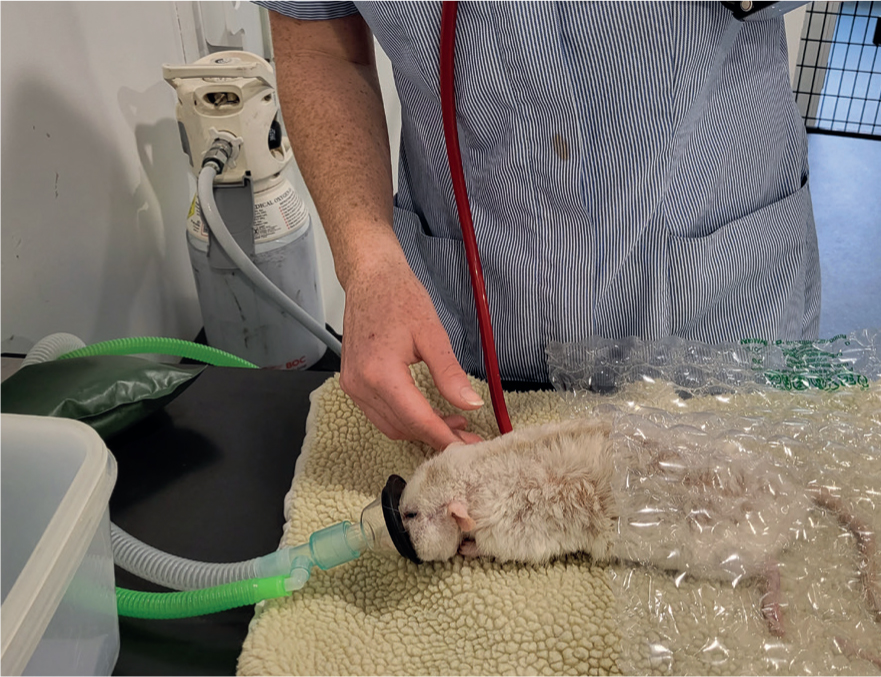
When anaesthetising small rodents, the use of an anaesthetic chamber is preferable as it reduces handling stress (Figure 3). Once the animal is anaesthetised remove it from the chamber and place a mask over its face attached to an Ayres T-piece anaesthetic circuit (Figure 4). As the lid of the chamber is removed there will be a leakage of anaesthetic gas which can risk the health of personnel treating the animal, it is important to use a well-ventilated room to reduce this. The authors recommend placing the animal in a clean induction chamber with some absorbent paper inside to soak up any urine, should urination occur during induction. This also helps minimise the risk of hypothermia, reducing the risk of the fur being soaked in urine as righting reflex is lost. The chamber is pre-oxygenated and then rapidly filled to reduce involuntarily excitement during induction, with a volatile anaesthetic agent (e.g. 5% isoflurane or 8% sevoflurane), while under constant visual monitoring of the rate and pattern of respiration. A reduction in respiration rate of more than 50% of normal should cause concern (Richardson and Flecknell, 2009). It is preferable to use sevoflurane, if possible because of the adverse smell associated with isoflurane and the increased risk of causing irritation to the mucous membranes (Guedes et al, 2017).


The time to fill a chamber can be calculated by dividing the volume of the chamber in litres by the flow rate, this will give the number of minutes. A chamber measuring 20 x 20 x 30 cm has a volume of 12 litres, using a flow rate of 4 litres/minute would take 3–4 minutes to fill. Induction chambers come in a variety of sizes and the smallest one possible should be used to reduce stress, as it will increase the speed of induction (Flecknell, 2009d).
Larger rats can be intubated with the use of a flexible videoendoscope and using commercially available rodent workstands and intubation packs (Richardson and Flecknell, 2009) but is technically challenging. Endotracheal intubation also narrows the effective airway, often necessitating ventilation in smaller rodents to avoid airway occlusion from respiratory secretions (Kubiak, 2021).
Maintenance of anaesthesia is often via a facemask with oxygen and 1.5–2% isoflurane or 3–5% sevoflurane (Richardson and Flecknell, 2009). Masks can be made out of syringe cases, if there is not a small enough one available.
If surgery of the head and neck is required, and the animal cannot be successfully intubated, inhalation agents and oxygen may be delivered via an intranasal catheter. Rodents are nasal breathers and in rats and mice, intravenous cannulas (with the stylet removed) can be used (Richardson and Flecknell, 2009). A small amount of bleeding may occur from insertion of the nasal cannula. Gas flow rates needed to maintain anaesthesia are similar to that required to prevent rebreathing when a facemask is used (three times minute volume). The disadvantage of delivering anaesthesia via a nasal catheter is that waste gas scavenging may be difficult, unless a downdraft table is used (Richardson and Flecknell, 2009).
Monitoring
The cardiovascular, respiratory systems and temperature should be monitored regularly, at least every 5 minutes or less, pre, intra and postoperatively until the rodent is fully recovered (Figure 5). During anaesthesia the patient must be constantly monitored. Lost reflexes can be monitored to assess anaesthesia depth. The first reflex to be lost is the righting reflex, eye position will not be a useful indicator in rodents so should not be used. The pedal withdrawal reflex and tail pinch reflex should be used in small rodents and can be indicative of a surgical plane. Care must be taken in gerbils when performing the tail pinch (Thompson and Bament, 2012). Doppler probes can be used to detect blood flow and provide an audible signal that can be assessed during the procedure. Doppler probe placement on the thorax may allow audible monitoring of both heart rate and respiratory rate for smaller patients (Bennett, 2022). Pulse oximetry can be used to indicate respiratory function, but some do not read heart rates over 250 beats per minute (bpm) so care must be taken with these. The probe can be attached to the tail or foot of the rodent. A sidestream capnograph unit may be used as this does not require an intubated patient to gain readings, however, it is better to follow trends of end tidal CO2 (ETCO2) readings looking at the character and frequency of breath as most sidestream units give falsely low readings because of low tidal volumes and high respiratory rates of the small rodent patient (Bennett, 2022).
Additional considerations
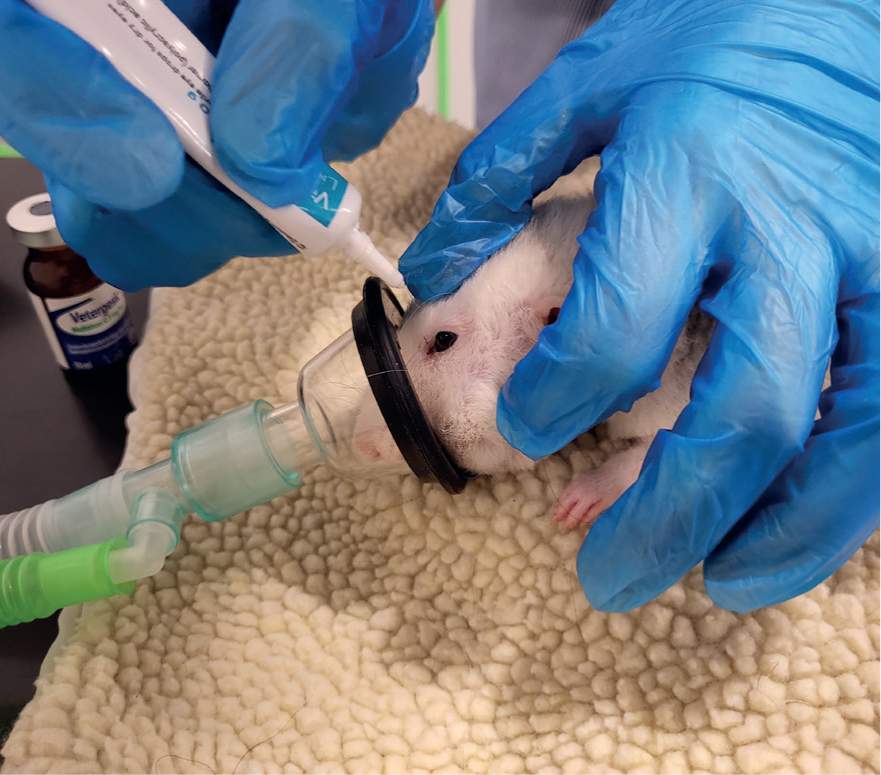
Ocular lubricant should be used to prevent dryness of the eyes during anaesthesia (Figure 6). If possible, position the rodent in sternal recumbency, with the chest elevated slightly higher than the abdomen (Thompson and Bament, 2012).
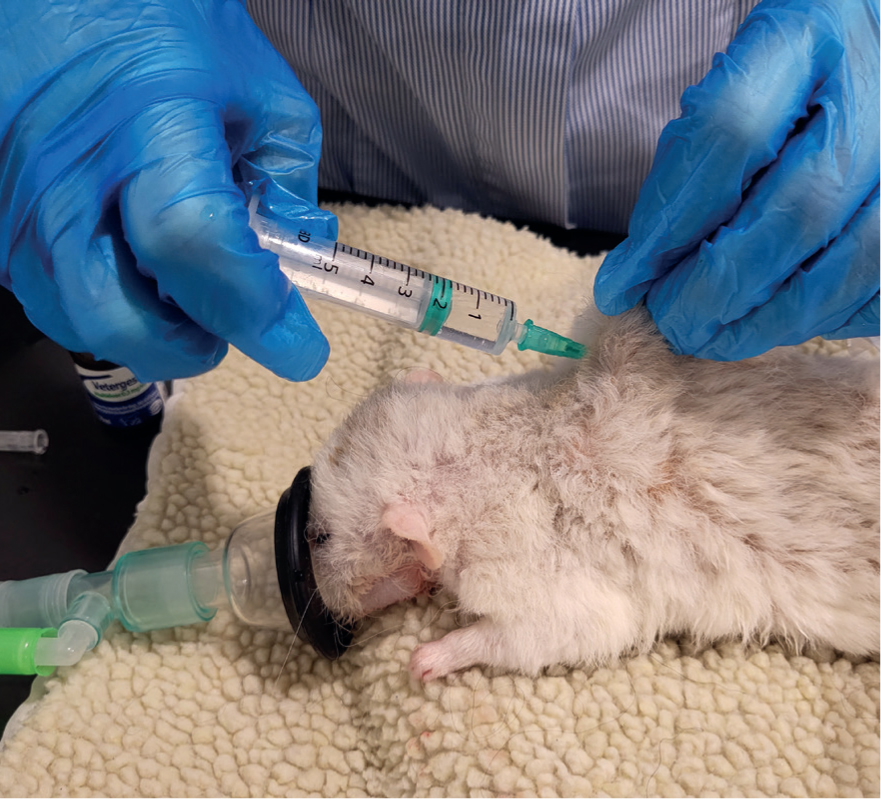
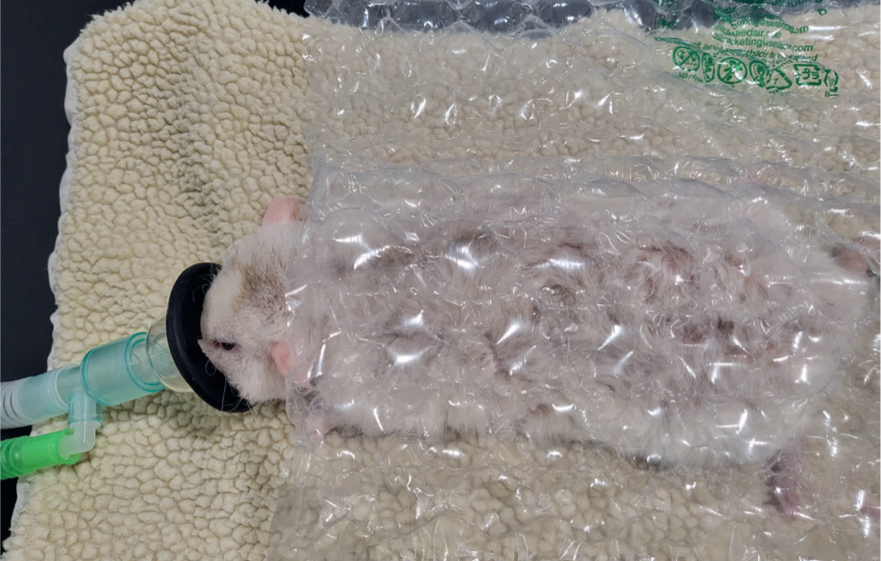
To minimise anaesthesia risks, it is important to administer analgesia suitable for the procedure, pre-oxygenate, give warmed fluids either via the intravenous route or if the rodent is small and if cannulation is unsuccessful, via the subcutaneous route (Figure 7) and keep the animal warm by placing it on a covered heat pad and wrapping as much of it up in bubble wrap, towels, and blankets as possible (Figure 8). Warming fluids can help preserve body temperature. As these rodents tend to be very small it can be difficult to access the animal when it is wrapped up. Bubble wrap and blankets/towels can be cut to size to assist with this problem. Care must be taken when wrapping a rodent so that respiration is not impeded (Flecknell and Richardson, 2009). Latex gloves filled with warm water can also be used to surround the rodent, ensuring that the gloves are secure, not placed directly on to the animal and checked regularly for leaks. It is strongly recommended that a temperature probe is placed between the heat source and the animal, and the rectal temperature is monitored to ensure that hyperthermia and thermal burns do not occur (Flecknell and Richardson, 2009). The area of fur clipped before surgery should also be minimised to reduce the risk of hypothermia.
Recovery period
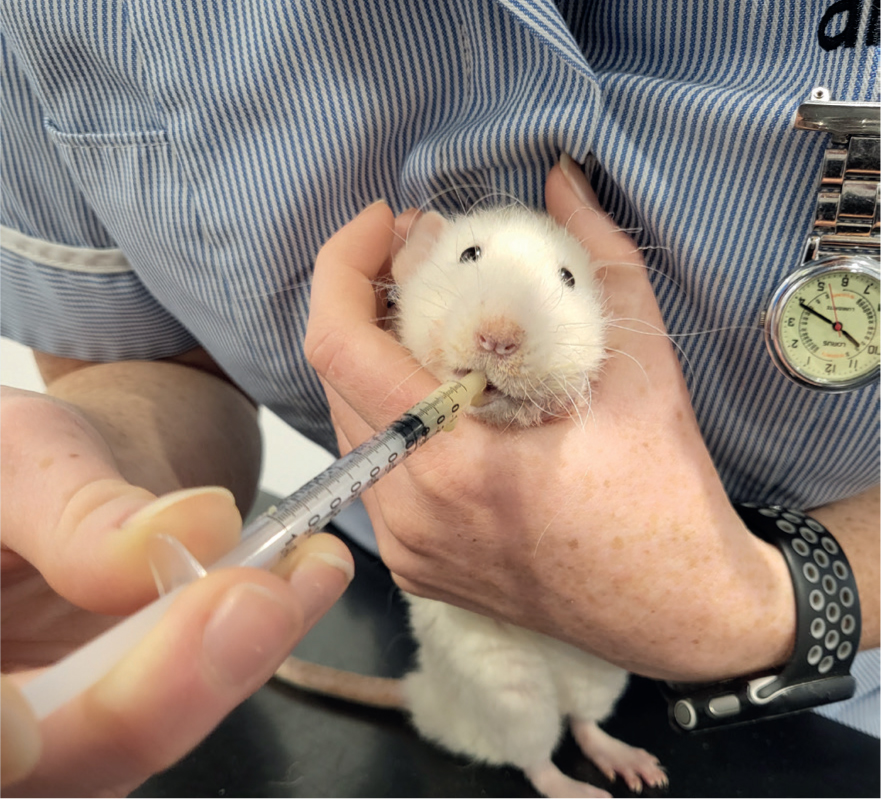
Patients need to be recovered in a warm, quiet and darkened room and, as they generally will not be initially eating and drinking as they should, assisted feeding and fluid therapy should be continued (Figure 9). A recovery cage should be provided that contains supplemental heat to maintain an ambient temperature of 26.6–29.4°C (Bennet, 2022). Analgesia should be continued for at least 3–5 days post-surgery.
Conclusions
To maximise the success of anaesthesia in rodents, the main factors to address are stabilisation and improvement of the clinical condition of the animal, prevention of hypoxia, prevention of hypothermia and administration of safe and effective analgesia.
KEY POINTS
- Anaesthesia of small rodents can be challenging and is associated with a higher risk compared with anaesthesia of dogs and cats.
- Small mammals have higher metabolic rates and smaller glycogen reserves predisposing them to hypoglycaemia.
- It is vital that an accurate bodyweight is taken in the clinical assessment.
- Hypothermia is a common complication in the anaesthetic period.
- To maximise the success of anaesthesia stabilisation is required, and prevention of hypoxia, hypothermia is essential.
- Multimodal analgesia is essential.


