Deepening the veterinary nurse's knowledge of pharmacology and mechanism of action (MOA) allows for a better understanding of drug choices, and subsequent monitoring, in an emergency situation. The veterinary nurse can often receive emergency patients arriving at the hospital with cardiac dysfunction. Cardiac emergencies can present with cardiogenic shock with or without congestive heart failure (CHF), cardiac arrhythmias, or in cats, paralysis as a result of aortic thromboembolism. Arrhythmias can be a result of underlying and pre-existing cardiac disease, systemic or infectious disease, trauma, electrolyte imbalance or pain. Medications discussed in this article pertain specifically to CHF, cardiogenic shock and cardiac arrhythmias.
Anatomy and physiology
The heart contains four chambers, the left and right atria and left and right ventricles. These four chambers receive and send blood through several vessels and valves to keep the body perfused with oxygenated blood and help remove carbon dioxide (Figure 1).
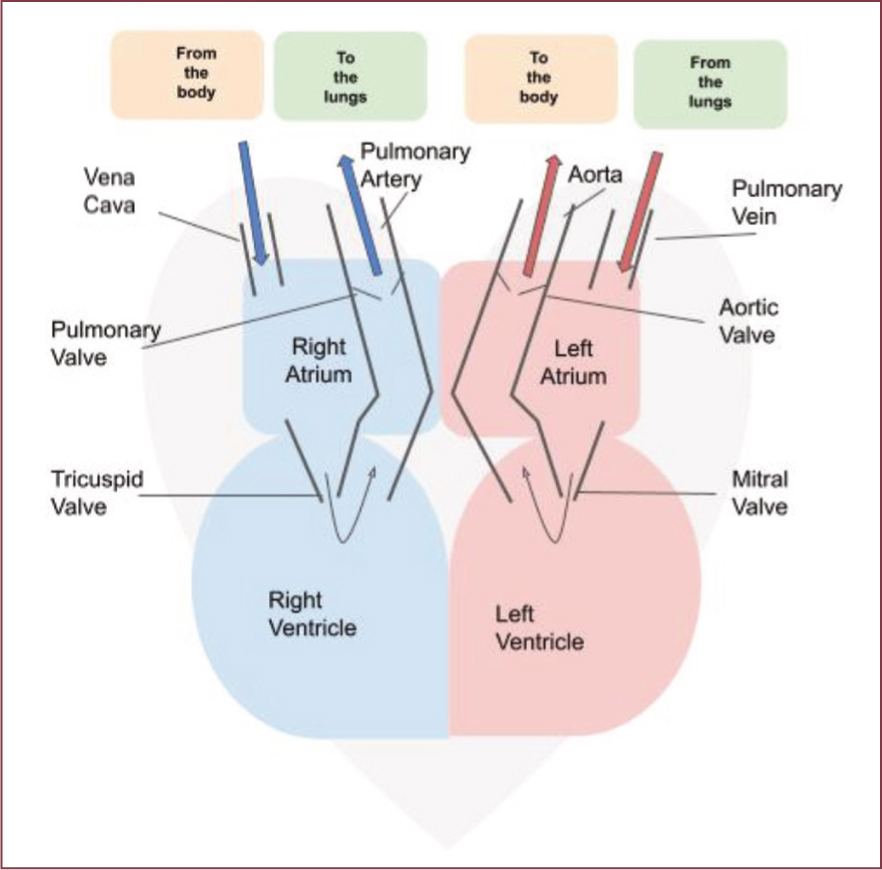
Deoxygenated blood comes into the heart from the body through the vena cava. This blood moves from the right atrium to the right ventricle through the tricuspid valve. It then flows to the lungs via the pulmonary artery where it will be oxygenated.
Oxygenated blood comes from the lungs back to the heart via the pulmonary veins into the left atrium and is then pumped into the left ventricle through the mitral valve. From the left ventricle, the blood is pumped out through the aorta to the rest of the body.
Any impediment along the way can lead to congestion of blood backing up to the right or left side of the heart, causing fluid build-up in the body's tissues.
Potential causes of heart failure in the emergency patient
CHF is said to occur when abnormal fluid accumulation is present. This happens when the heart is unable to meet the metabolic needs of the body and activates compensatory mechanisms. In the short term, these mechanisms can be lifesaving, but in the long term can have deleterious effects. The extra fluid can create higher pressures, which in turn can lead to pulmonary or venous congestion. Forward or output failure can occur when compensatory mechanisms do not have time to activate, which is seen with arrhythmias or end stage disease causing diastolic dysfunction. Table 1 shows the more common causes of heart failure. Box 1 discusses the differences between preload and afterload.
Table 1. Causes of heart failure can be categorised into four groups
| Cause | Definition |
|---|---|
| Systolic dysfunction | A problem in the pumping action of the heart. An example of this is dilated cardiomyopathy (DCM) |
| Diastolic dysfunction | The ventricles cannot relax and fill properly. Examples of this are hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM)Obstructive dysfunction falls within this category because it also impedes filling. An example of this is pericardial effusion with cardiac tamponade |
| Volume overload | Due to anatomical problems, either congenital or acquired, blood volume can back up and cause congestion (systemic and/or pulmonary). Examples of volume overload disorders are myxomatous mitral valve disease (MMVD), patent ductus arteriosus, or some ventricular septal defects |
| Pressure overload | This occurs when the ventricles need to produce a higher than normal systolic pressure to eject blood. Examples of pressure overload disorders are pulmonary or aortic stenosis, and pulmonary or systemic hypertension |
Box 1.What does preload and afterload mean?Afterload is the pressure the heart must overcome for the left ventricle to pump blood into the aorta and subsequently, around the bodyPreload is a measure of the ventricular stretch or ventricular volume at the end of diastole (relaxation). As the volume increases, the volume ejected at contraction also increases. If the volume is too high venous congestion and/or oedema could occur(Ware, 2011)
Common medications used in the treatment of acute heart failure and congestive heart failure
Guidelines in the form of consensus statements have been published in recent years to help manage heart failure patients with myxomatous mitral valve disease (MMVD) (Keene et al, 2019) and feline cardiac disease (Luis Fuentes et al, 2020). Box 2 details the nursing recommendations that have been made. Alongside the nursing considerations, pharmacological intervention is also required. Common medications used in an emergency setting include:
- Furosemide (diuretic)
- Pimobendan (positive inotrope and vasodilator)
- Dobutamine (positive inotrope)
- Nitroprusside (venodilator).
Box 2.Acute stabilisation of the heart failure patientRecommendations for acute stabilisation of the cardiac patient have come from the American College of Veterinary Internal Medicine (ACVIM) consensus statements on myxomatous mitral valve disease (2019) and hypertrophic cardiomyopathy (2020). These recommendations include:
- Hospitalisation for cage rest and close monitoring. Environment should be quiet and as stress free as possible
- Oxygen therapy
- Careful and considered handling of dogs and cats, to avoid increasing stress
- Optimal nursing care, including changing litter trays or bedding if soiled, providing water, monitoring respiratory rate and effort. Managing access needed to the patient, to ensure the patient can rest and benefit from optimal oxygenation
- Furosemide intravenously (IV) or intramuscular (IM), if IV access is not possible
- Opiate sedation as necessary
- Preparation for thoracocentesis if required
- Other medications as directed by the veterinary surgeon
Furosemide
Furosemide is a loop diuretic. It has a fast onset of action when given intravenously (IV), and can also be given subcutaneously, intramuscularly and per os (Plumb, 2018). Furosemide is indicated when abnormal fluid accumulation is suspected or confirmed. For life-threatening cases of pulmonary oedema, furosemide may be administered as a continuous rate infusion (CRI) after initial bolus (Keene et al, 2019).
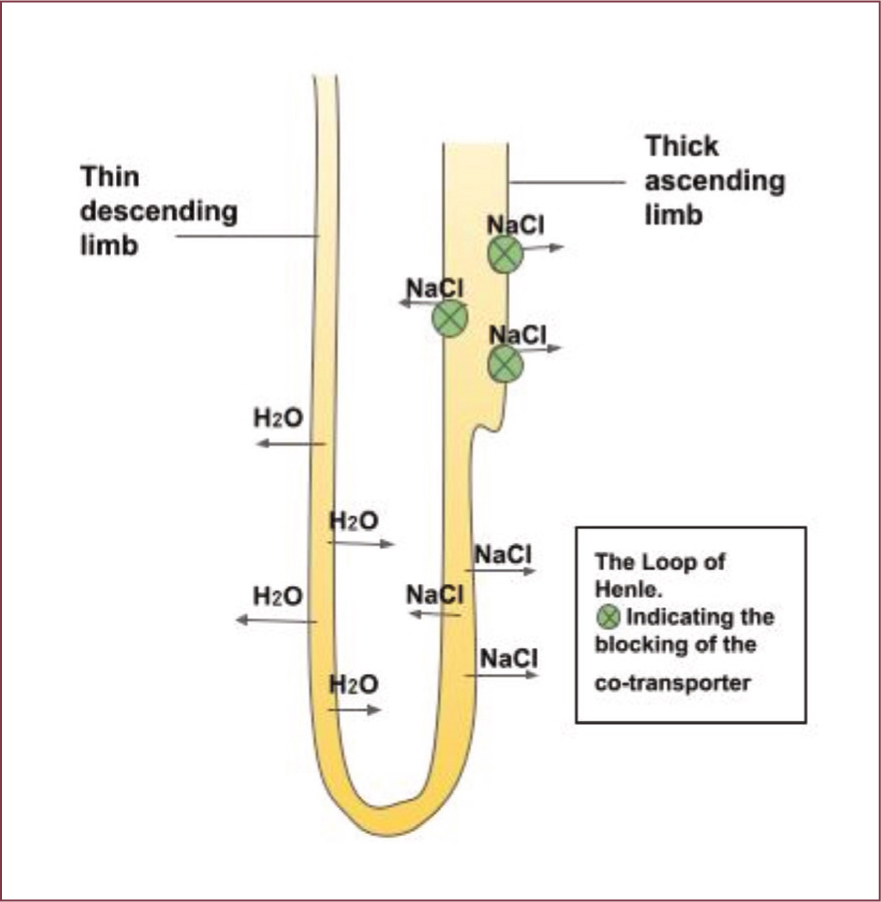
- MOA — furosemide works by blocking the sodiumpotassium-chloride (Na+-K+-Cl-) co-transporter in the ascending loop of Henle, this results in decreased reabsorption of sodium and chloride leading to increase in natriuresis and diuresis (Figure 2) (Francey, 2015).
- Side effects — side effects of furosemide include dehydration, renal injury and hypokalaemia.
Pimobendan
Pimobendan is a positive inotrope and an arterial vasodilator. Thus, it increases cardiac contractility and reduces afterload by making the heart pump more effectively. It can be given IV or per os. IV administration is preferable in the emergency patient as it has a faster onset, reduces the stress caused while giving or ‘pill-popping’ oral medication, and is more readily absorbed. Shock can cause impaired circulation and as a consequence, the gut may be inadequately perfused, causing less reliable absorption of per os medication. Pimobendan is recommended for use in dogs with pre-clinical dilated cardiomyopathy (DCM) and MMVD, and CHF. In cats, it is used in cases of heart failure, but is currently not licensed (Pace, 2019).
- MOA — pimobendan is a calcium sensitiser. It allows troponin C to be sensitive to the prevailing level of calcium in the cell but does not increase the level of calcium. Troponin C then increases its interaction with contractile proteins and acts as an inotropic agent. It also works as a balanced vasodilator through various mechanisms which result in a decrease in the amount of calcium in the smooth muscle of the vessel, and this causes vessel relaxation (dilation).
- Side effects — poor appetite, lethargy, diarrhoea, dyspnoea, azotaemia, and weakness (Ware, 2011).
Dobutamine
Dobutamine is a positive inotrope. It increases contractility strength, and therefore cardiac output. Dobutamine is given IV via a CRI and is useful in increasing forward flow and cardiac output (Haskins, 2015). It is particularly helpful in the treatment of cardiac shock (Reynolds and Hochman, 2008), and is recommended in cases with MMVD and acute heart failure (Keene et al, 2019). Blood pressure and electrocardiograph (ECG) monitoring is advised during administration (Plumb, 2018).
- MOA — dobutamine stimulates the beta-1 adrenergic receptors and to a lesser extent, beta-2 receptors. This results in increased contractility with limited effect on heart rate. Caution is required with administration of dobutamine, as it can have proarrhythmic effects.
- Side effects — tachyarrhythmias or arrhythmias, and central nervous system (CNS) symptoms in cats (Dubin et al, 2017).
Nitroprusside
Nitroprusside is a potent vasodilator. It must be given as a CRI and covered, as it is light sensitive (Plumb, 2018). Its use in veterinary medicine is generally reserved for the treatment of critically ill patients with conditions such as hypertensive crisis (Acierno et al, 2018) and acute heart failure secondary to MMVD (Keene et al, 2019) or DCM (Ware, 2011). It is used only when constant blood pressure and ECG monitoring can be performed, hence nitroprusside is not commonly used in practice. Administering nitroprusside will reduce both afterload and preload, because of its ability to dilate both arteries and veins, and aids rapid left ventricle unloading (Novo Matos and Summerfield, 2018). It may be used synergistically with dobutamine (Plumb, 2018).
- MOA — sodium nitroprusside breaks down in circulation to release nitric oxide (Friederich and Butterworth, 1995). Through various further mechanisms, nitrous oxide causes vascular smooth muscle relaxation, allowing vessel dilation.
- Side effects — side effects include cyanide toxicity, metabolic acidosis, seizures and stupor (Labato, 2015). It should not be administered for more than 48 hours. (Novo Matos and Summerfield, 2018).
Cardiac conduction system
The conduction system is the mechanism that is responsible for initiating and coordinating the heart's contraction and relaxation. In essence, it is the system that instigates blood to be pumped around the body. The cardiac conduction system consists of a group of specialised cells that send signals to the heart muscle causing it to contract. The main components of the cardiac conduction system are the sinoatrial (SA) node, atrioventricular (AV) node, bundle of His, bundle branches, and Purkinje fibres. The SA node is the pacemaker, or initiation point, and creates an electrical impulse, known as an action potential, which travels through the heart via the electrical pathway, resulting in contraction of the atria (Figure 3). As the impulse passes through the AV node there is a slight delay, as the impulse cannot pass through all at once. This delay allows the atria to contract just before the ventricle, ensuring the atria have enough time to fully eject blood into the ventricles before starting ventricular contraction. Box 3 summarises the conduction pathway.
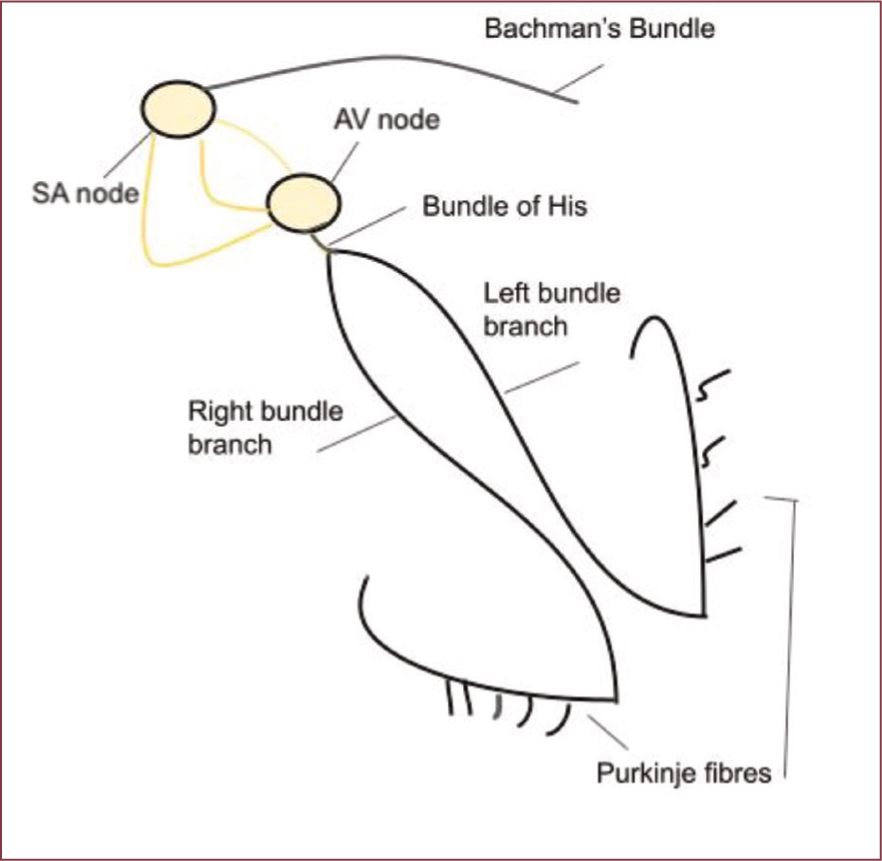
Box 3.Summary of the conduction pathwaySinoatrial (SA) node → left atria (via Bachman's bundle)SA node → atrioventricular (AV) nodeAV node → bundle of His (left and right bundle) → Purkinje fibres (in the ventricles)
Action potential
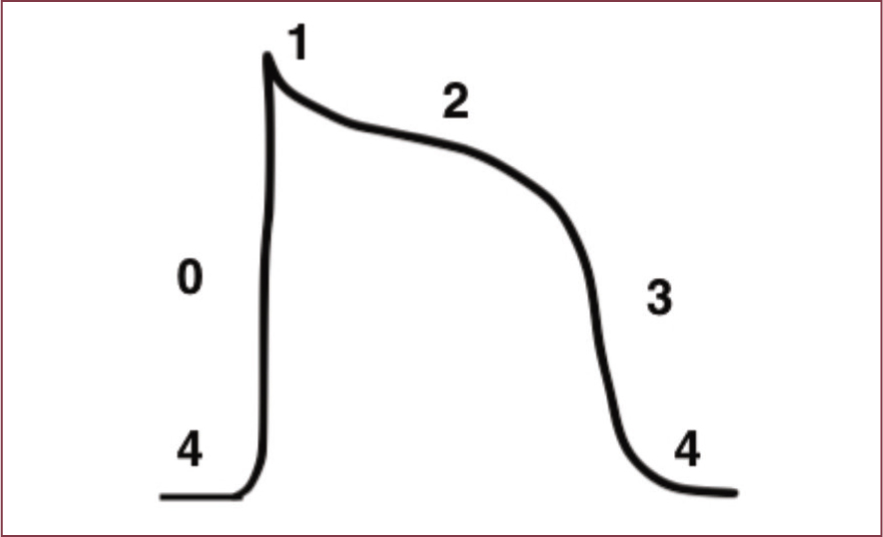
Cardiac action potential depicts the rapid change of membrane potential within a cardiac myocyte. Action potentials occur in association with changes in cell membrane permeability to sodium (Na+), potassium (K+) and calcium (Ca+) ions. Transmembrane movement of these ions depends on the opening and closing of ionspecific channels. This causes a depolarisation or repolarisation of a cell. The action potential varies depending on the specific type of cardiac cell from which it arises, for example a cell within the SA node, or a ventricular myocyte. An action potential has five phases: 0, 1, 2, 3, 4. Antiarrhythmic drugs work at different phases of the action potential, and may be more effective on one type of myocyte than another (Wright, 2015a). Figure 4 shows an action potential.
Potential causes of cardiac arrhythmias in the emergency patient
The development of abnormal cardiac rhythms is dependent on several factors, some of which are listed in Table 2. Some cardiac arrhythmias may have little or no clinical consequence, but others may cause weakness, syncope or death. An ECG is vital for the identification and diagnosis of arrhythmias.
Table 2. Potential causes for cardiac arrhythmias
| Underlying cardiac disease | Myxomatous mitral valve disease (MMVD), hypertrophic cardiomyopathy (HCM) or dilated cardiomyopathy (DCM). Less common causes are myocarditis, conduction abnormalities and endocarditis |
| Physiologic and pathologic alterations | Sympathetic nervous system stimulation (high sympathetic tone), including hypovolaemia, fear, painParasympathetic nervous system stimulation, such as vagal nerve stimulationSystemic disease, examples are endocrine disease, sepsis, intra-abdominal disease (gastric dilation-volvulus, splenic rupture, pancreatitis) or anaemia |
| Drug side effects or toxicities | Beta-blocker overdose/ingestion, alpha-2 agonist overdose, opioids given at inappropriate doses. Other intoxications that may cause cardiac disturbances are theobromine, cannabinoids and amphetamines (Dolder, 2013; Volmer, 2013) |
| Electrolyte imbalance | Potassium imbalance may be seen in the emergency patient and can cause cardiac arrhythmiasCalcium imbalance, among other electrolyte disturbances, can also result in cardiac disturbances (Schenck et al, 2011) |
Antiarrhythmic drug classifications
Antiarrhythmic medication is categorised into four groups using the Vaughan Williams classification system.
Class I
- Inhibit sodium channels
- Class I is subdivided into:
- Ia — depresses phase 0 depolarisation and prolongs repolarisation
- Ib — depresses phase 0 depolarisation in abnormal tissue
- Ic — significantly depresses phase 0
- Lidocaine is classed as Ib.
Class II
- Beta-adrenergic antagonists (beta-blocker)
- Blocks the beta-adrenergic receptor from increasing heart rate
- Acts to slow down ventricular rate
- Includes esmolol and atenolol.
Class III
- Prolong repolarisation
- Potassium channel blockers
- Includes sotalol and amiodarone.
Class IV
- Block the (non-dihydropyridine) calcium channels
- Useful in AV node related tachyarrhythmias
- Includes diltiazem.
Common medications for the emergency treatment of arrhythmias
It is important to remember that all antiarrhythmic drugs have the potential to have pro-arrhythmic effects. Therefore, during administration, the patient should be monitored carefully. ECG and blood pressure monitoring are recommended. The antiarrhythmic drugs discussed in this article are limited to lidocaine (Ib), sotalol (III) and diltiazem (IV), as these are the more common drugs used in practice. The type of an antiarrhythmic treatment will depend on the origin of the arrhythmia, and the clinical symptoms (Ware, 2011).
Lidocaine
Lidocaine is a commonly used antiarrhythmic drug in practice. It is the first choice for acute treatment of ventricular arrhythmias (Figure 5) (Pariaut, 2015). Lidocaine is an IV agent. To see an immediate effect, lidocaine is administered initially as a slow bolus (over 2 minutes). Thereafter more boluses can be given, or it can be used as a CRI (Plumb, 2018). Without a loading dose, steady-state plasma concentration occurs only after 4–6 hours (Ware, 2011). The effectiveness of lidocaine is enhanced during acidosis and where there is an increased amount of extracellular potassium (Wright, 2015b). When hypokalaemia is present the opposite is true, and the antiarrhythmic effect of lidocaine can be significantly reduced (Ware, 2011).
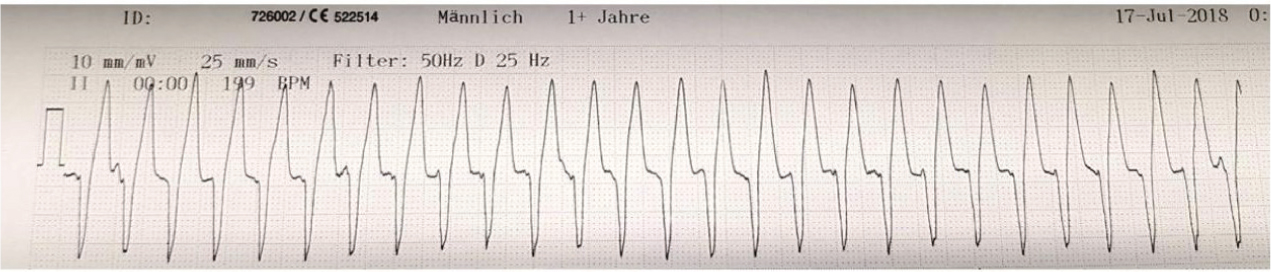
- MOA — lidocaine is a Ib antiarrhythmic agent. It is a fast sodium channel blocker and works at phase 0 of the non-pacemaker action potential, where there is an influx of sodium. Inhibiting sodium channels makes the heart less likely to initiate or conduct early action potentials that may cause an arrhythmia.
- Side effects — include gastrointestinal signs such as nausea, vomiting and excessive salivation. Toxicity may also manifest as tremors and seizures. Lidocaine is metabolised by the liver. Any interference with hepatic clearance could result in toxicity. Lidocaine should be used with caution in cats. It has been reported that cats are sensitive to lidocaine, with side effects being reported in the CNS (Plumb, 2018).
Sotalol
Sotalol can be used in the treatment of ventricular or supraventricular arrhythmias and comes in IV or tablet form. Because of its potential to act as a negative inotrope, blood pressure monitoring is especially important if used IV.
- MOA — sotalol is a class III antiarrhythmic agent with beta-blocker properties. It prolongs the refractory period (the period of time where the cardiac cell is unable to initiate another action potential), by selectively blocking the rapid component of K+ channels responsible for repolarisation.
- Side effects — adverse effects of sotalol may include hypotension, depression, nausea, vomiting, diarrhoea, and bradycardia (Ware, 2011).
Diltiazem
Diltiazem is often prescribed for the treatment of supraventricular tachycardia. It is available for use as an IV injection or in tablet form (Plumb, 2018).
- MOA — diltiazem is a class IV Ca+ channel blocking cardiac antiarrhythmic agent. The use of Ca+ channel blockers increases the minimum time between action potentials, or the refractory period, in the AV nodal cells, and slows the sinus rate (Ware, 2011).
- Side effects — can include gastrointestinal problems, hepatopathy or inappetence. It can also cause skin lesions or rashes. In extreme cases, hypotension, bradycardia and dyspnoea can occur (Noah Compendium, 2021).
Conclusion
The veterinary nurse should feel encouraged to understand medications commonly administered to the emergency cardiac patient. Gaining a deeper knowledge and understanding about these medications will hopefully not only increase the level of patient care, but also help the veterinary nurse in supporting the veterinary surgeon, ultimately allowing greater job satisfaction.
KEY POINTS
- The patient arriving with a cardiac emergency may be in cardiogenic shock, congestive heart failure, arrhythmia or have an aortic thromboembolism.
- The veterinary nurse is encouraged to be familiar with the drugs used in their practice, and gain a deeper knowledge and understanding of the medications they administer.
- A deeper knowledge will hopefully result in higher patient care standards and increased job satisfaction.


