Different species of ascarids and ancylostomatids, commonly called ‘roundworms’ and ‘hookworms’ respectively, infect the small intestine of dogs and cats in Europe. These parasites are significant to human and animal health, as they are the most important nematodes affecting companion animals globally, in terms of infection rates, geographical distribution and risks to both animal and human health (Traversa, 2012; Morelli et al, 2021).
In Europe, dogs are infected by the roundworm Toxocara canis and the hookworm Ancylostoma caninum, while cats can be infected by T. cati and A. tubaeforme. Less commonly found nematodes such as Toxascaris leonina and Uncinaria stenocephala can infect dogs and, to a lesser extent, cats (Bowman et al, 2010; Traversa, 2012). The raccoon roundworm Baylisascaris procyonis is mostly prevalent in North America, but has been detected in central and northern countries of Europe (Gavin et al, 2005; Popiołek et al, 2011; Al-sabi et al, 2015).
T. canis and T. cati are the primary global ascarid infections in dogs and cats respectively. These nematodes have a complex life cycle, characterised by different pathways of larval migration and transmission, depending on different factors, eg source of infection and the age of the animal (Traversa, 2012). The vertical transmission is a pivotal aspect for the life cycle of T. canis. In dogs, bitches are the primary reservoir of infection for puppies, which occurs through the reactivation of hypobiotic somatic larvae in the tissues, leading to subsequent transmission to the offspring via both the placenta and milk. Conversely, prenatal transmission of T. cati does not occur and lactogenic transmission only happens if a queen acquires the nematode during late pregnancy (Traversa, 2012). Dogs and cats of all ages can contract the infection by ingesting embryonated Toxocara eggs in the environment (these ova remain infective for years) or by consuming the tissue of paratenic hosts (eg ruminants, rodents or birds), both of which are a constant source of infection (Bowman et al, 2002; Bowman, 2009; Epe, 2009; Lee et al, 2010).
T. leonina can affect both dogs and cats and is generally less widespread than other roundworm species, as animals only acquire this infection by ingesting developed eggs from the environment or larvae within paratenic hosts tissues (eg rodents), with no vertical transmissions (Epe, 2009). Dogs become infected with B. procyonis by either ingesting larvated eggs from the environment or consuming infective larvae present in paratenic hosts, particularly rodents (Lee et al, 2010; Sapp et al, 2020). Dogs are the only non-procyonid animal species susceptible to B. procyonis, and serve as both definitive hosts, developing patent intestinal infections, and paratenic hosts, experiencing clinical larva migrans (Yabsley and Sapp, 2017; Heller et al, 2019). Dogs infected by B. procyonis might pose a greater risk to human health than raccoons as they tend to defecate indiscriminately, while raccoons typically have designated areas known as ‘latrines’ for defecation (Page et al, 1998).
The hookworms A. caninum, A. tubaeforme and U. stenocephala can be found both in warmer and colder regions of temperate and subarctic zones across both hemispheres. Other hookworm species are found in subtropical and tropical countries (Manning et al, 2006; Palmer et al, 2007; Traub et al, 2007; Bowman et al, 2010). Hookworms also have a complex biological cycle, with different sources and routes of infection. Larvae in the soil penetrate the skin of the host and are the primary route of transmission, particularly in the case of Ancylostoma spp., or larvae can be transmitted orally (Anderson, 2000; Bowman, 2002; Epe, 2009). In dogs, somatic larvae of A. caninum can be re-activated by stress-inducing factors, such as severe illness, treatments involving corticosteroids, oestrus and pregnancy. As a result, larvae migrate to the intestine, leading to patent infections in adult dogs, while in pregnant bitches these larvae can be transmitted via the milk to the litter for at least 3 weeks post-partum (Bosse et al, 1980; Stoye, 1992; Bowman et al, 2002; 2010). Vertical transmission does not seem to occur for the other hookworm species affecting dogs and cats, but paratenic hosts (eg rodents) play a significant role in transmitting ancylostomosis to dogs and cats during predation activities (Anderson, 2000; Bowman et al, 2002).
Puppies and kittens usually show higher parasitic burdens and egg outputs, but dogs and cats of all ages are exposed to roundworms and hookworms throughout the year.
Clinical importance in dogs and cats
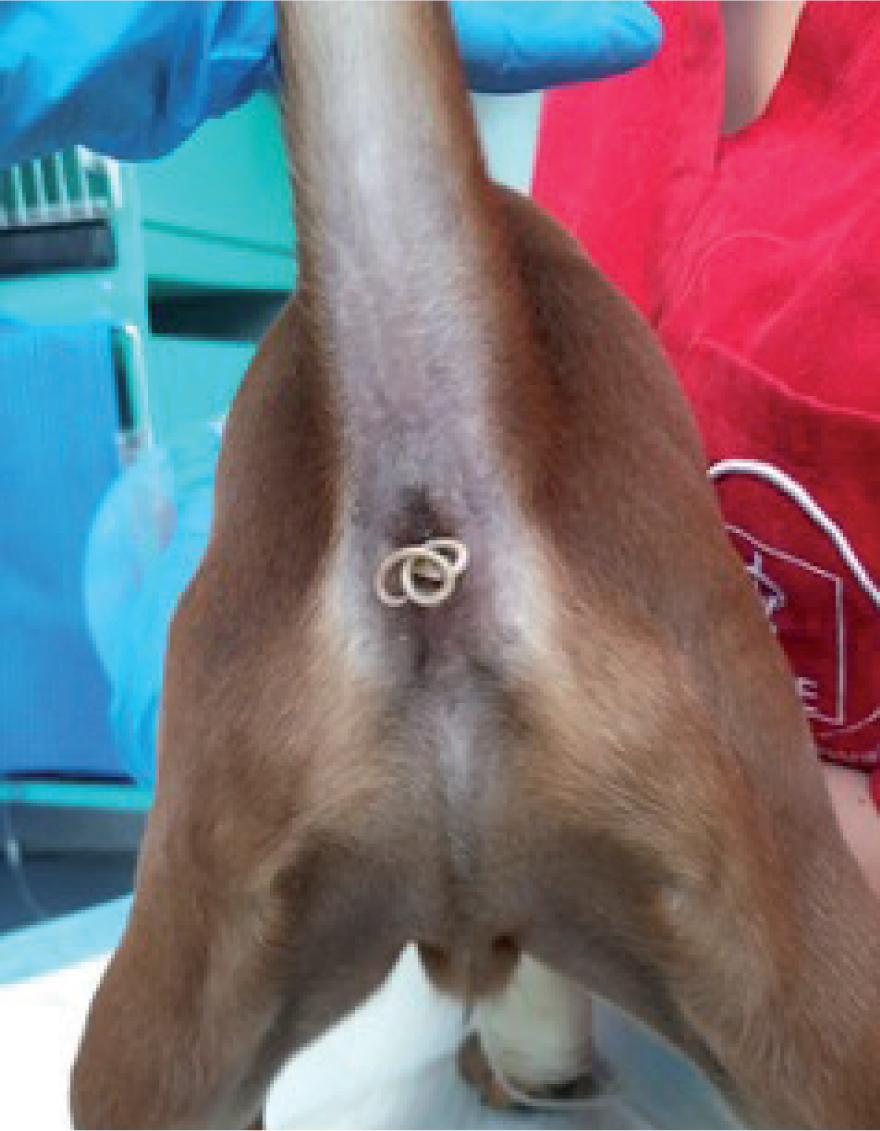
Roundworms reside within the lumen of the small intestine, feeding on nutrients within it. The clinical picture in infected animals depend on different factors related to the host (eg age) or to the parasite (eg parasitic burden). In puppies and kittens, adult roundworms cause a mucoid enteritis with vomiting, diarrhoea, poor growth, loss of appetite, emaciation, ascites and a distended abdomen. These clinical signs arise as a result of a substantial worm burden, dysbiosis (microbial imbalance), and the formation of gas within the intestine (Hendrix, 1995; Eckert, 2000; Bowman et al, 2002; Bowman, 2009; Epe, 2009). Often animals spontaneously expel adult worms with diarrhoea and vomitus (Figure 1) (Bowman et al, 2002; Bowman, 2009; Epe, 2009). Severe infections can result in the death of puppies and kittens because of various complications such as intestinal obstruction, dilation of the duodenum, peritonitis, intestinal rupture, haemorrhage, or obstruction of the bile and pancreatic ducts. Larval migration can also lead to pneumonia in young animals, with cough, nasal discharge, dyspnoea and death, especially shortly after birth, in puppies born infected through transplacental transmission (Dade and Wiliams, 1975; Epe, 2009). T. leonina is usually less pathogenic in both dogs and cats and causes similar, but milder, signs.
Hookworms are among the most pathogenic nematodes of dogs and cats. They live anchored to the intestinal mucosa feeding on the host blood, causing different clinical pictures varying from a mild enteritis to a fatal hemorrhagic diarrhoea, depending upon different factors, such as the age of the animal, parasitic burden and species involved (Bowman et al, 2002; Bowman, 2009). U. stenocephala tends to induce mild disease, although it can contribute to intestinal disease alongside other coinfections, whereas Ancylostoma spp. cause potentially life-threatening disease, especially in puppies and kittens, as a result of significant blood loss (Kalkofen, 1987; Traversa, 2012). Clinico-pathological alterations more commonly observed are haemorrhagic diarrhoea, iron deficiency or anaemia, weight loss to cachexia, reduced growth, circulatory collapse and lethargy (Epe, 2009; Traversa, 2012).
Threat to human health
Most roundworms and hookworms of dogs and cats have the potential to infect humans. T. canis iss a significant zoonotic parasite across the globe, while further research is being undertaken to fully understand the extent of the role of T. cati in human infections (Traversa, 2012; Rostami et al, 2019). Nevertheless, studies suggest that Toxocara spp. eggs are more prevalent in sandpits than in park soil, indicating that T. cati could be the most prevalent roundworm in urban settings (Mizgajska-Wiktor et al, 2017; Tyungu et al, 2020). Humans become infected through ingestion of eggs in the environment or from a paratenic host. A frequent source of human infection is also food, particularly vegetables harvested from farms that use animal dung as fertiliser, or from the consumption of raw or undercooked meat from animals such as ruminants, pigs or chickens (Stürchler et al, 1990; Salem and Schantz, 1992; Lee et al, 2010).
Direct contact with an animal infected by roundworms might not be deemed hazardous for several reasons. Toxocara eggs take about 2–6 weeks to become infective, they adhere strongly to the animal's fur making ingestion challenging, most eggs on hair are non-viable, and finally, a significant amount of fur would need to be swallowed to cause an infection (Overgaauw, 1997a, 1997b; Overgaauw and van Knapen, 2004; Overgauuw et al, 2009; Keegan and Holland, 2010; Nagy et al, 2011).
Once infective elements are ingested, Toxocara spp. larvae reach different tissues and organs via the bloodstream, do not moult to adult, and cause local reactions and lesions, known as larva migrans syndromes. While certain infections might not display any clinical signs, two primary syndromes can emerge: visceral and ocular larva migrans syndromes. Visceral larva migrans involves mainly liver and lungs and it is more commonly observed in children, causing severe diseases, eg pneumonitis, necrotic hepatitis, myocarditis, meningoencephalitis, seizures, and neuropsychiatric signs (Overgaauw, 1997b; Despommier, 2003). Ocular larva migrans affects the optic nerve, causing severe consequences, such as glaucoma, detachment of the macula, total loss of sight or ocular alterations mimicking a retinoblastoma with unnecessary eye enucleations (Despommier, 2003; Bowman, 2009). Other syndromes have been reported (eg long-term exposure to migrating larvae in different tissues), although these are of less clinical and epidemiological importance.
T. leonina is not zoonotic, while B. procyonis is probably the most pathogenic zoonotic ascarid. The close proximity of raccoons to human and pet populations poses a significant health risk in certain areas. This is because infective eggs of B. procyonis remain viable for years, serving as a source of infection for wildlife, paratenic hosts and dogs (Wise et al, 2005). However, the contribution of dogs to the occurrence of human larval syndromes caused by B. procyonis remains uncertain. Humans become infected when they inadvertently swallow infective eggs. The larvae cause a severe neural larva migrans syndrome, and can also lead to ocular or visceral larva migrans, and diffuse unilateral neuroretinitis. Although there are few documented human neural larva migrans cases from B. procyonis, its pathogenic nature emphasises the crucial need to control infections in domestic animal populations. Given the permanent and life-threatening cerebral damage caused by the parasite, preventive measures are crucial (Graeff-Teixeira et al, 2016).
Different species of ancylostomatids have different zoonotic and epidemiological importance. Human infection occurs when third-stage larvae (L3) present in the soil penetrate the skin, leading to cutaneous lesions that range from local irritation to the development of a cutaneous larva migrans syndrome. In the last decade, the involvement of zoonotic hookworms in the occurrence of human diseases has been subject to debates (Bowman et al, 2010; Traversa, 2012). A. braziliense is responsible for cutaneous larva migrans in humans, resulting in characteristic dermatitis with serpiginous tracks on the skin. The disease is endemic in (sub)tropical regions of the southern hemisphere and, even though suspected cases of cutaneous larva migrans have been reported in Europe, the aetiological agents causing this remain unknown (Bowman et al, 2010; Blaizot et al, 2017; Gutiérrez García-Rodrigo et al, 2017; Del Giudice et al, 2019).
Hence, the actual contributions of A. caninum, A. tubaeforme and U. stenocephala as causative agents of cutaneous lesions and cutaneous larva migrans are yet to be fully understood. The potential for A. tubaeforme and U. stenocephala to infect humans is doubtful, as they exhibit minimal capability to penetrate human skin (Bowman et al, 2010; Traversa, 2012). In contrast, A. caninum is capable of inducing localised lesions, such as papular or pustular eruptions, rather than the characteristic serpiginous tracks commonly associated with cutaneous larva migrans (Caumes et al, 2002; Malvy et al, 2006; Rivera-Roig et al, 2008). Moreover, A. caninum has been associated with causing unilateral subacute neuroretinitis, eosinophilic enteritis and myositis (Hunter and Worth, 1945; Prociv and Croese, 1990; Garcia et al, 2008; Jung et al, 2020).
Diagnosis
Faecal floatation technique is the gold standard for the diagnosis of a wide range of parasites in dogs and cats, including intestinal nematodes. This is a commonly used and easy to perform test that helps detect and identify eggs of hookworms and roundworms in stool samples. A small amount of the faecal sample is mixed thoroughly with the flotation solution until a homogeneous faecal suspension is obtained. The mixture is filtered through a double layer of cotton gauze to remove large debris or material that might interfere with microscopic examination. The mixture is then poured into a falcon tube and centrifuged. After the centrifugation, a floating solution is added to the top of the tube to obtain a meniscus on which a coverslip is placed. After 3–5 minutes, the coverslip is put on a microscope slide and observed at low–medium magnification (Dryden et al, 2005; Blagburn and Butler, 2006). Collection and examination of three faecal samples could increase the sensitivity of the test because the excretion of the eggs can be discontinuous.
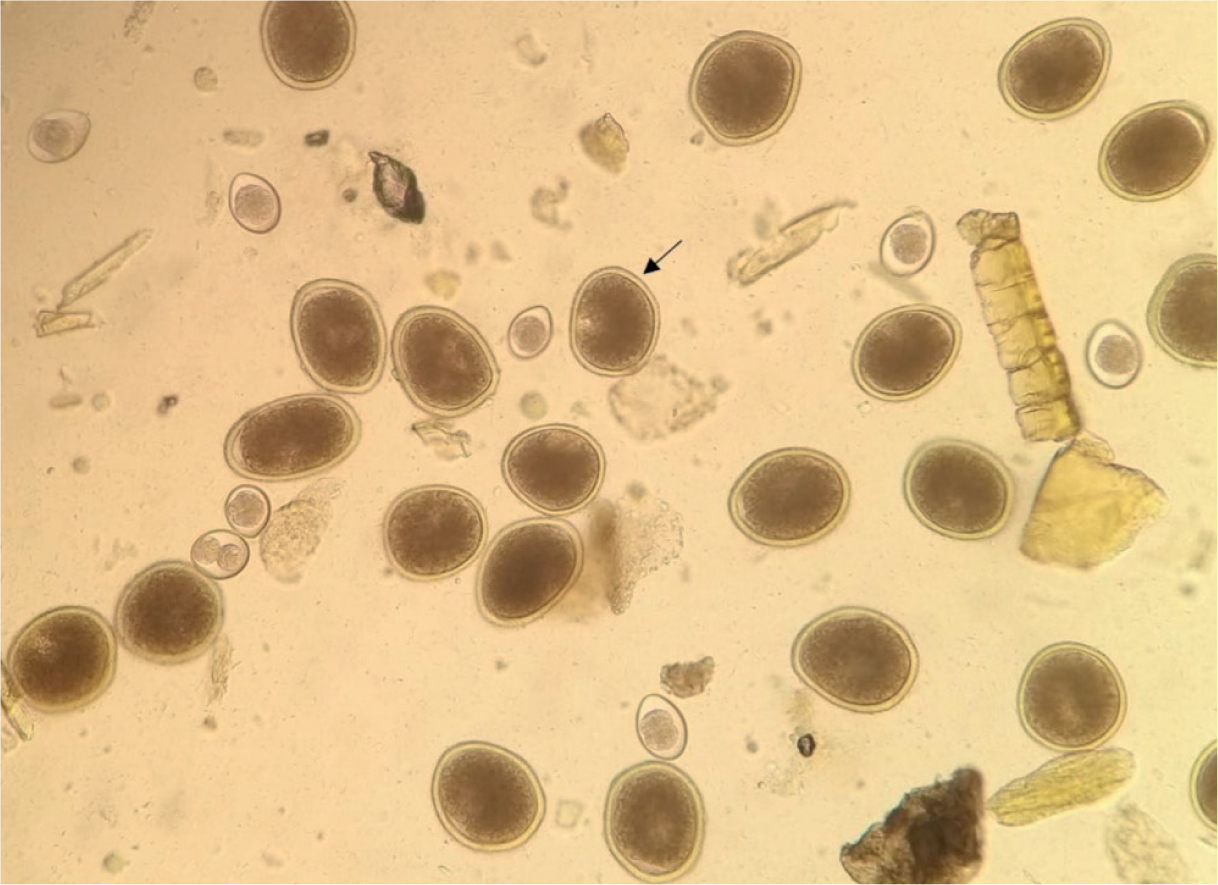
Eggs of T. canis, T. cati and T. leonina are usually present in high quantities and easily identified (Figure 2). However, in certain regions (eg North America), the raccoon roundworm B. procyonis can pose diagnostic challenges because the eggs resemble those of T. canis (Kazacos, 2001, 2006; Traversa, 2012). Therefore, in areas where B. procyonis is prevalent, veterinary practitioners need the expertise and training to distinguish B. procyonis eggs from those of T. canis. This is crucial for safeguarding the public health of individuals potentially exposed to faeces from dogs infected by B. procyonis. The potential misdiagnosis of B. procyonis and Toxocara spp. is a substantial risk, with serious implications for public health, especially in Europe because of the lack of awareness of this parasite by veterinary practitioners.
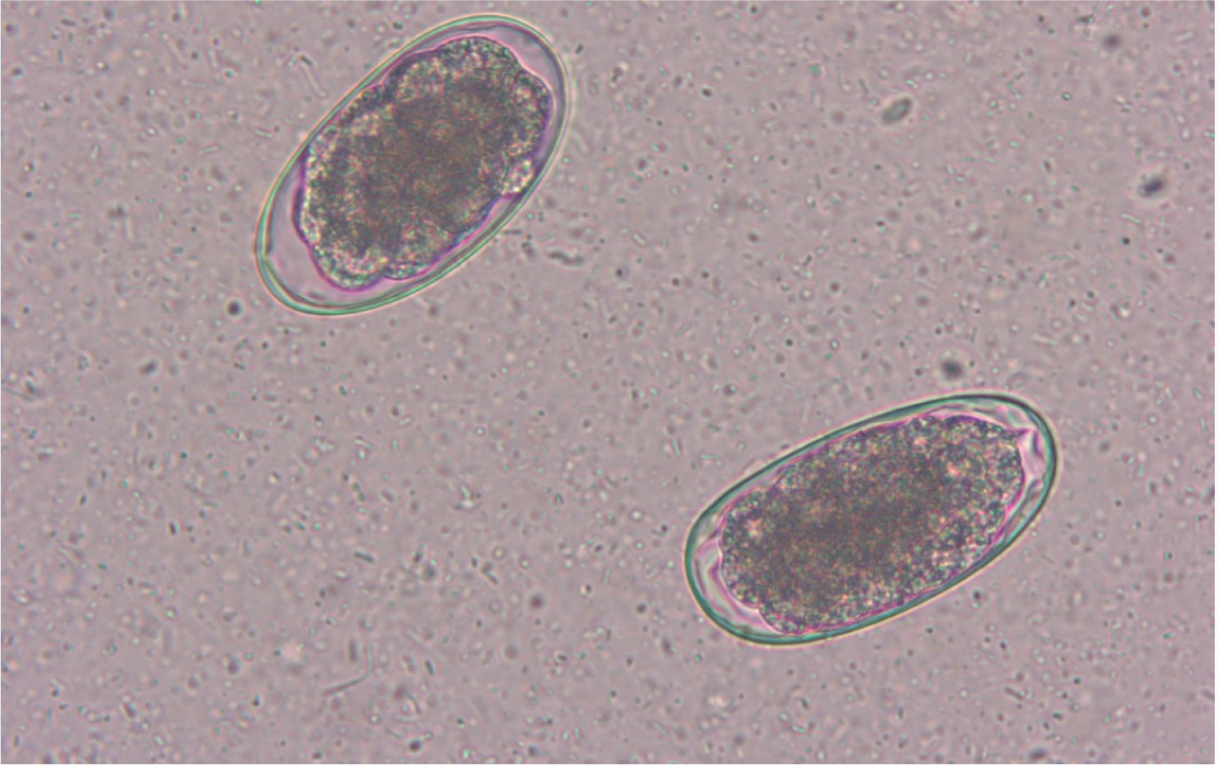
It is difficult to identify hookworm infection in dogs and cats at the species level with certainty, because eggs shed by the different species have similar and shared morphological and morphometric characteristics (Figure 3). While coprocultures can be conducted for a precise diagnosis, in practical terms, the presence of hookworm eggs in the faeces of pets would require parasitic treatment even without the confirmation of the species affecting the animal.
Copromicroscopic examinations should be routinely performed in dogs and cats, as they are consistently exposed to potential re-infection by roundworms and hookworms throughout their lives. Moreover, faecal floatation should be conducted regardless of the presence of gastrointestinal signs like diarrhoea or vomiting, as studies have shown no substantial variation in nematode infections between animals showing clinical signs and those without them (Gates and Nolan, 2009a, 2009b; Savilla et al, 2011). On the other hand, infected animals pose a similar level of zoonotic risk even when clinically healthy. It is important to conduct regular faecal examinations throughout the life of the animal, and for the veterinary practitioner to inform the owner about the importance of performing faecal examinations more than once in a year, depending on age, lifestyle and frequency of antiparasitic treatment (Stehr-Green and Schantz, 1987; McCarthy and Moore, 2000; Traub et al, 2005; Katagiri and Oliveira-Sequeira et al, 2008).
Newer diagnosis methods include faecal antigen testing, faecal polymerase chain reaction and artificial intelligence aids for faecal flotation.
Control measures
The control of roundworms and hookworms in pets relies on an integrated approach to reduce infection and prevent the spread of these parasites. The cornerstone of successful prevention lies in ensuring that both pet owners and the general public are aware and well-informed. Additionally, ongoing education for veterinarians and technicians plays a pivotal role in providing pet owners with precise information, scientific support, deworming schedules, details about the effectiveness of the several products available on the market, and everyday measures suitable for the wellbeing of pets.
There are currently no practical methods to entirely remove infective elements of intestinal nematodes, particularly ascarids, once they are present in the environment. Therefore, preventing the initial contamination of environments is a primary objective to mitigate the spread of these parasites.
The outdoor presence of infective parasitic elements often stems from a lack of education and awareness among pet owners of the actual zoonotic risks involved. Areas commonly used by both children and pets, such as backyards, sandpits, parks, playgrounds, and beaches, pose a significant risk for heavy contamination with pet faeces (Despommier, 2003; Holland and Smith, 2006; Lee et al, 2010; Mizgajska-Wiktor et al, 2017; Tyungu et al, 2020). Therefore, basic preventive measures, essential for all pet owners to know, include preventing animals from defecating in public spaces, or consistently cleaning up animal faeces from the ground. Educating pet owners about the regular removal and proper disposal of faeces, along with emptying cat litter trays, is crucial. These practices are vital in reducing environmental contamination and lowering the risk of transmission, benefiting both animals and humans alike (Overgaauw, 1997b; Robertson and Thompson, 2002).
Favourable environments for the survival and growth of free-living larvae of hookworms include shaded, warm, humid and well-drained soils. Preventing both animal and human ancylostomosis requires proper hygiene measures such as regular hand and feet washing, disposing of pet faeces, wearing shoes and avoiding contact with areas where animals commonly defecate or areas with a high likelihood of contamination. While travelling in regions at risk for cutaneous larva migrans, travellers should avoid walking barefoot on beaches or lying directly on the sand, particularly since A. braziliense is endemic in many popular tourist spots, and they might return home with the infection at the end of their holiday.
Conclusions
Roundworms and hookworms poses significant health risks to both animals and humans. Understanding the routes of transmission, such as contaminated environments, and the potential consequences for human and animal health, including potentially life-threatening conditions, highlights the need for continuous education among pet owners and the public. Veterinary professionals play a crucial role as educators, providing accurate information on preventive measures, deworming schedules and environmental hygiene.
Efforts aimed at preventing initial contamination of environments, promoting responsible pet ownership, and fostering awareness about the zoonotic risks associated with these parasites are essential. Implementation of these measures may minimise the spread of roundworms and hookworms and mitigate their impact on both animal and human health. Continued research, education and collaborative efforts are fundamental in safeguarding the wellbeing of both pets and people against these significant zoonotic threats.

