Gastric dilatation volvulus (GDV), sometimes called gastric torsion, is a known emergency condition in dogs, although other species have been documented to suffer from this condition including monkeys, ferret, swine and humans (Dudley, 2011). In GDV, the stomach becomes dilated with gas and twists on its axis. This increases intragastric pressure and in turn causes compression of the gastric vessels causing ischaemic damage to the tissues of the stomach and spleen. The direct pressure of the dilated stomach on other vessels also causes portal hypotension and obstructive shock from the vena cava becoming compressed, thus reducing venous return to the heart and decreasing cardiac output. Without corrective treatment this condition leads to death. Mortality rates in dogs vary, but cases which are managed surgically have a higher rate of survival, one recent study suggesting 79.3% of surgical cases survived to discharge (O'Neill et al, 2017). Other studies have suggested survival rates as high as 90% in a referral centre setting, but this may not reflect general practice presentations.
The signalment in canine patients is usually large and giant breeds of dog (commonly documented breeds include Great Danes, Setters, Weimaraners and Basset hounds). The underlying cause of GDV in dogs is poorly understood. There appears to be many factors which may be involved in the development of this condition, these include increased age, once daily meals, rapid eating, an anxious temperament and an increase in thoracic depth to width radio (Gazzola and Nelson, 2014), which increases the risk in certain breeds. The many clinical signs within dogs include unproductive retching, progressive abdominal distention, restlessness, tachycardia and signs of hypovolaemia (Fossum, 2006)
The signalment for guinea pigs with this condition is not fully known. It is likely to have multiple factors, a few reports on GDV in these species has showed that females that were either gravid or have had previous pregnancies have suffered from this condition, these animals were in a laboratory setting. Pet guinea pigs reported are of both genders and some with no history of breeding. It could be assumed that previous breeding could be an associated risk factor, but further research needs to be performed. Other suggestions include increased risk with feeding fewer meals a day, rapid eating, competition, increased stress after a meal, fearful temperament and age (Nogradi et al, 2017), but more work needs to be done to show if these factors are to be of any substantial risk.
Few to no presenting signs have been documented in guinea pigs and sudden death has been reported in many cases. Other clinical signs are suggested to be similar to signs of gut stasis in guinea pigs. These clinical signs are often non specific (DeCubellis et al, 2013). One report of two sudden deaths due to GDV showed the two patients had lost 9% and 30% of bodyweight 7 days prior to death (Dudley et al, 2011). The author has also witnessed a sudden death of a 5-year-old entire female of their own, a post mortem revealed GDV with disseminated intravascular coagulation (DIC) the cause of death, the guinea pig appeared well with no clinical signs or weight loss prior to death. A report on six guinea pigs seen in a breeding colony showed clinical signs of tachycardia, dyspnoea, cyanosis and marked abdominal distention (Lee et al, 1977), and a pet guinea pig living alone had anorexia and abdominal distension for 24 hours prior to elective euthanasia (Mitchell et al, 2010). This highlights the difficulty in diagnosing GDV in guinea pigs and may be a factor contributing to the high mortality rate quoted in the few papers reporting the condition.
Treatment for GDV in guinea pigs is the same as that of dogs and requires critical care and corrective surgery to return the stomach to the normal position. A paper documenting eight guinea pigs presented at an exotics specialist clinic that received surgery for GDV showed a 75% mortality rate (one euthanased due to necrotic tissue damage, one died during surgery and four 0–1 day post surgery). The two surviving patients survived for 94 days and over a year (alive at date of article publishing) (Nogradi et al, 2017). This shows that even when these patients are recognised and given emergency care there is a high risk of mortality.
A case report
Susie is an approximately 2–3-year-old female entire guinea pig, and is a Lakeland breed of guinea pig (this is a gene carrier for skinny hairless breed). The patient had been used to breed from multiple times by her previous owner. This was evident in her body physique with her abdominal wall having been stretched, giving her a pear shaped appearance. She housed with ten other individuals, nine other sows of various ages and a neutered boar. Her diet consists of an unlimited supply of meadow hay with restricted pellets and a variety of mixed vegetables and herbs once a day. Her enclosure in an approximately 37 square foot indoor run with fleece bedding and hay in litter trays used as substrate. Prior to any clinical signs the patient had remained well and in the owner's possession for the past year, her weight had remained stable and her last pregnancy had been approximately 15 months prior.
20 days prior to being presented for a GDV the patient showed vague clinical signs at home. She had reduced appetite and was reluctant to interact with her companions. Over the course of a 24-hour period she developed hypersalivation, soft faeces and acute mid abdominal pain. These symptoms were managed at home under a veterinary surgeon's guidance and after 48 hours of supplemental syringe feeding and meloxicam the guinea pig returned to usual activity and eating habits. However over the coming 20 days she remained quieter than usual and her weight reduced. A full clinical examination 3 days after initial clinical signs revealed no abnormalities, she was monitored at home and was given time away from the group to self feed sporadically.
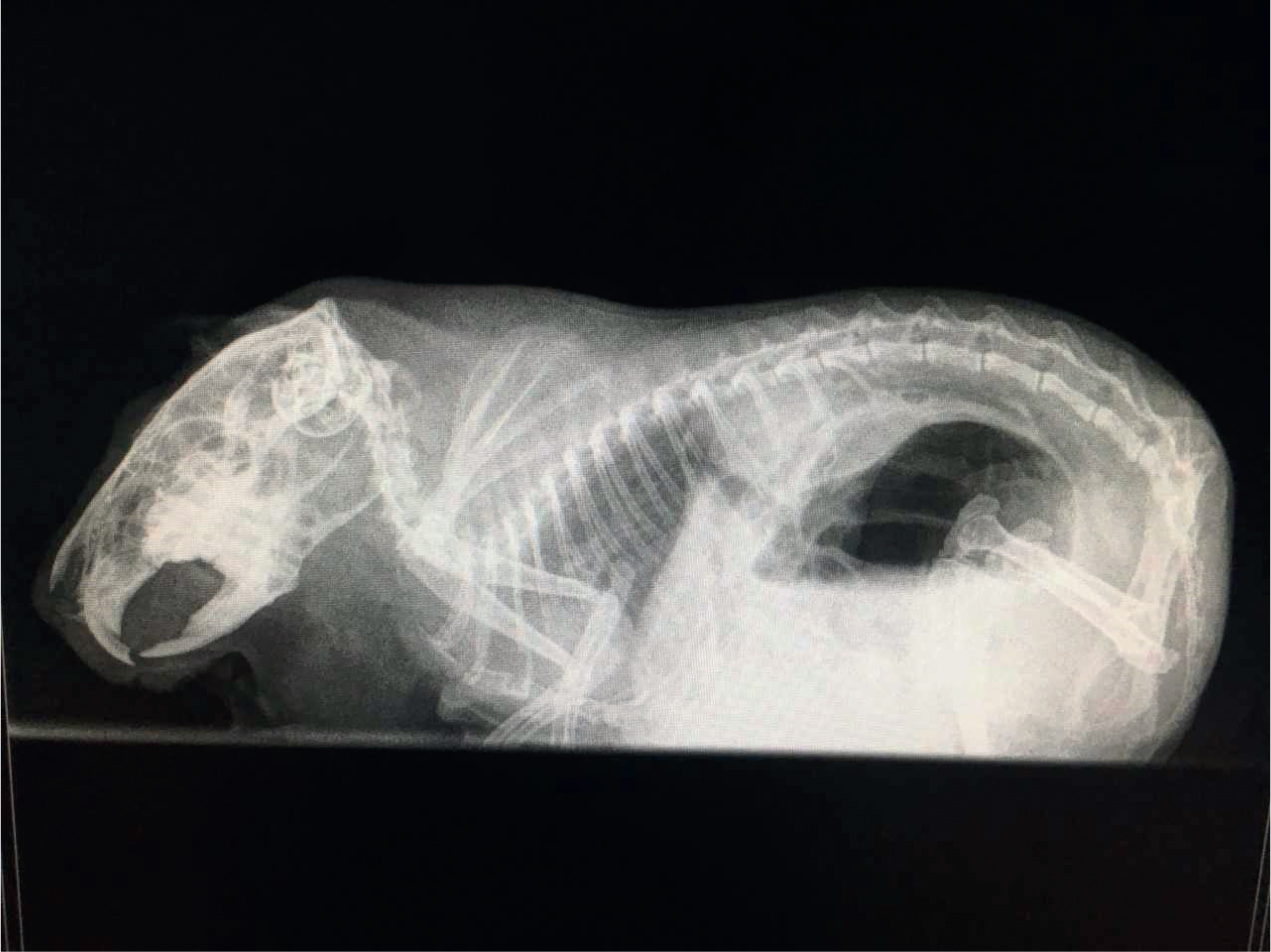
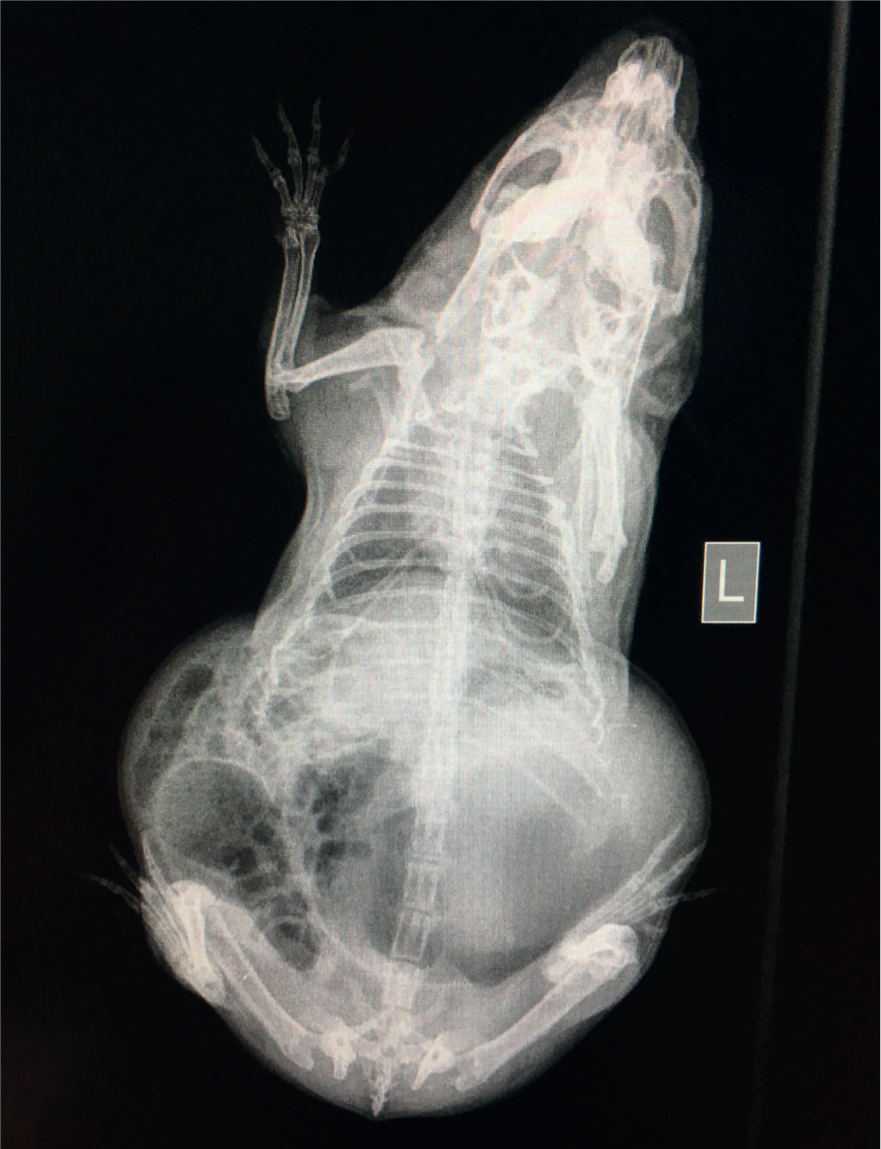
After 20 days the patient developed an acute onset of hypersalivation and retching early in the morning, the owner had been asleep so the duration of these signs is unknown but less than 7 hours. The guinea pig was unable to swallow and at this point was presented at the veterinary hospital as an emergency for further investigation. On arrival, her temperature was 36.1°C (normal range (37.2–39.5°C), heart rate 240 beats per minute (bpm) (normal range 240–310 bpm) and respiratory rate 80 breaths a minute (normal range 40–120). Abdominal palpation elicited a retching response and she was very reactive to mid to caudal abdominal palpation. Her stomach was large and gassy but not tympanic and her caecum felt slightly gas filled, however this was difficult to assess fully as her pain levels were high during examination. She was given an injection of meloxicam (Metacam cat, Boehringer Ingelheim Ltd) at 0.6 mg/kg and buprenorphine (Buprecare, Animalcare UK) at 0.05 mg/kg subcutaneously and an injection of midazolam (Hypnoval, Roche) at 0.2 mg/kg intramuscularly to provide analgesia and anxiolysis. She was also given a subcutaneous injection of ranitidine at 4 mg/kg to provide a pro kinetic to help move the gas through the gastrointestinal tract. Two conscious radiograph images, a right lateral via horizontal beam and a dorsal-ventral were obtained. These revealed gas filled caecum and stomach with an odd pattern on the image consistent with gastric dilation and potential volvulus but they were inconclusive (Figures 1a and 1b). It was at this point that it was decided that an exploratory laparotomy would be in the best interests of the patient. While awaiting surgery the patient was placed into an incubator set at 30°C due to her hypothermia. In rabbits, hypothermia on presentation at a veterinary clinic has a direct correlation to mortality (Di Girolamo, 2016); this is probably due to a low temperature being a consistent finding with patients that are suffering from systemic shock and hypovolaemia. At this point taking the patient's blood pressure would have been ideal, however due to their size and temperament it is tricky to take Doppler readings on conscious guinea pigs and it was not attempted in this case. Care should be taken when taking blood pressures in smaller patients as the size of the cuffs are often too large for the species, leading to falsely low pressure readings (Hawkins et al, 2007). Attention should be paid to trends of blood pressures in exotic species.
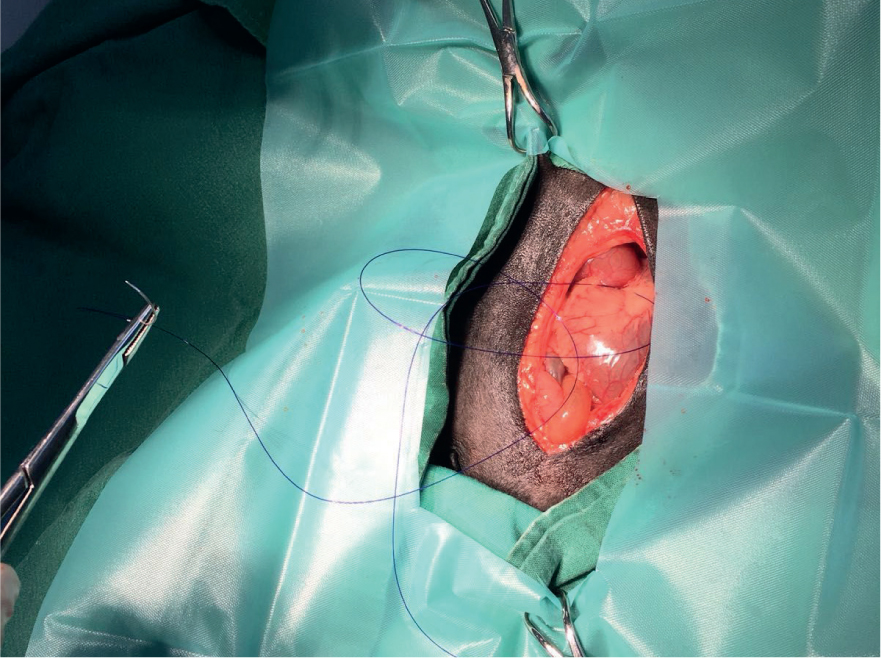
The guinea pig was taken to theatre where she was induced using 5% isofluane in a small gas chamber. She was maintained under general anaesthetic using a tightly fitted mask over the nose and with isoflurane at 2–3% during the procedure. The ventral aspect of the abdomen was clipped. It was aseptically prepared using undiluted 4% chlorhexidine gluconate surgical scrub solution (HiBiScrub® Mölnlycke Health Care). Practice policy is that the surgical scrub solution is not diluted, as recommend by the manufacturer. Preliminary studies have shown that only 21% of nurses are aware of the concentration of their surgical scrub (Evans, 2009) and dilutions less than 0.5% are likely to reduce antibacterial efficiency so care should be taken when preparing surgical sites aseptically (Figure 2). A 26G intravenous catheter was introduced into the left cephalic vein using a cut down technique. In the author's experience using a cut down technique has a better success rate at placing a catheter into guinea pig veins than without. An infusion of lidocaine at 0.05 mg/kg/minute was started via a syringe driver (Vet Pro SP 3000, Caesarea Medical Electronics Ltd.), this was run at a 4 ml/kg/hour rate which is maintenance rate for guinea pigs (Varga et al, 2012). The patient had cardiac arrhythmia and ventricular premature complexes (VPC) present on the echocardiography (ECG) trace. Around 40% of dogs suffering from gastric dilatation volvulus develop ventricular arrhythmias, likely due to ischaemic damage to the myocardium and cardiogenic shock (Sharp and Rozanski, 2014). Lidocaine is indicated in the presence of VPCs (Egger, 2016), it was continued throughout the procedure. Although arrhythmic the guinea pig's heart and respiratory rate remained otherwise stable throughout the procedure. However, there was significant hypothermia consistent with hypotension and shock. The ambient temperature of the theatre was maintained at 30°C and both hot hands and a bair hugger were applied to the patient to aid warming throughout the surgery. The stomach was noted to be cold on examination and the gastric vessels and the spleen were engorged and inflamed. The tissue of the stomach was still pink but was pale in colouration due to lack of blood supply, the spleen was on the left aspect of the abdomen rather than the right. The stomach had therefore twisted approximately 180° in a clockwise direction. This was carefully corrected and the gas within the stomach decompressed as the organ was placed back into a natural position. The stomach was then stitched to the abdominal wall to create a gastropexy using monofilament suture (Figure 3). Gastropexy was performed as it has been shown to reduce the chances of GDV reoccurrence (Rawlings et al, 2002). The abdomen was closed with a simple continuous layer and intradermal sutures. At this point the patient's arrhythmia had almost completely resolved. However the rectal temperature had now dropped to 34.6°C despite active warming.
Once the isoflurane gas was switched off the anaesthetic circuit was flushed and the patient was maintained on 2 litres/minute oxygen flow via the face mask. Low post operative temperatures can increase recovery time and mortality (Pottie et al, 2007) so she was actively warmed using a warm air unit (AAS Darvell Cocoon Warm Air Unit) for approximately 30 minutes in theatre (Figure 4). Her temperature increased to 35.4°C and at this point she was managing to lift her head. She was then moved to the designated small mammal exotic ward into an incubator set at 30°C where she was able to stand 40 minutes after the procedure and was normothermic 95 minutes post operatively. 2 hours after the procedure the patient received a bolus of high fibre critical care syringe feed (Oxbow, Critical care). As with rabbits, guinea pigs are hind gut fermenters and require a high fibre diet, initiating enteral feeding quickly is important as it increases motility, reduces pain associated with colic and aids normal gut flora (Orosz, 2013).
The guinea pig was maintained on the following drugs postoperatively: buprenorphine (Buprecare, Animalcare) 0.05 mg/kg subcutaneously every 6–8 hours depending on pain scoring; ranitidine (Zantac syrup, Glaxosmithkline) 4 mg/kg every 12 hours orally; cisapride (Cisapride 5 mg/ml solution, Summit Veterinary Pharmaceuticals) 0.5 mg/kg every 8 hours orally; meloxicam (Loxicom for dogs, Norbrook) 0.6 mg/kg every 12 hours orally; and Oxbow critical care syringe feeds 15 ml every 4 hours (with an 8 hour rest period overnight) (Table 1). She was given time with a companion during the daytime and separated between midnight and 8 am to monitor urine/faecal output and food intake.
Table 1. A summary of post-operative treatments
| Drug/treatment | Dosage and route | Frequency |
|---|---|---|
| Buprenorphine | 0.05 mg/kg, subcutaneous | Every 6–8 hours |
| Ranitidine | 4 mg/kg, per os | Every 12 hours |
| Cisapride | 0.5 mg/kg, per os | Every 8 hours |
| Meloxicam | 0.6 mg/kg, per os | Every 12 hours |
| Oxbow critical care feed | 15 ml bolus | 5 times a day (every 4 hours) |
Pain scoring was based on two scales which have been created for the hospital, using both the grimace scale (Rabbit grimace scale, Pain and Animal Welfare Sciences Group) which has been validated for rabbits, another has been created for rats and mice, and also a behaviour scale which was created by the author (Table 2). This is based on both personal experience and various literature on behavioural signs of pain in rabbits and rodents (Malik and Leach, 2017). Ensuring adequate analgesia in postoperative periods and periods of gut stasis in herbivores is essential to aid recovery as pain has been shown to increase stress levels and recovery times. Multimodal use of analgesia has been shown to provide better relief from pain in guinea pigs and other species (Oliver et al, 2017).
Table 2. Rabbit and rodent pain scoring
| Pain score | 0 | 1 MONITOR | 2 REASSESS PAIN RELIEF | 3 REASSESS PAIN RELIEF | 4 IMMEDIATE VET ATTENTION |
|---|---|---|---|---|---|
| Grimace scale |
|
|
|
|
|
| Behaviour |
|
|
|
|
|
Veterinary nurses must be familiar with the appearance and behaviour of the species and their individual patient's temperament before scoring. Stress can be associated with many of these behaviours such as tachypnoea and anorexia
The patient returned home late the following day post surgery. She had not eaten within the hospital but began to eat fresh herbs on the journey home. On arriving home she was separated into a small recovery cage close by to the group and bedded on a vet bed to monitor urine and faecal output. She began to eat both fresh vegetable and herbs as well as hay and grass almost immediately. Buprenorphine was continued 8–12 hourly under veterinary surgeon direction at home, this was stopped after 24 hours as the patient did not show any sign of discomfort and was happy for gentle abdominal palpation. She was maintained on 2–3 syringe feeds daily for a week to help maintain gut motility and weight. Cisapride was stopped 2 days after discharge and ranitidine 5 days after discharge due to a good faecal output. She was given 0.6 mg/kg dose of meloxicam for 10 days postoperatively. Over the 10 day period she was slowly integrated back into the herd, spending some time in a pen with a grid divide initially so she could eat without competition and be offered more food than others (Figure 5). Once back into the group she remained slightly quieter and more reserved than usual but within 14 days had resumed normal activity for her personality and was communally eating with the herd. The wound closed well however the patient did develop some suture reaction postoperatively, she was given a 5 day course of sulfamethoxazole trimethoprim (Septrin Paediatric Suspension, Glaxosmithkline) to cover for any infection but the swelling slowly reduced and no further issues were seen. It is common for rabbits and rodents to develop suture reactions, the risks can be reduced by using a monofilament suture, which have been shown to cause less inflammation (McFadden, 2011). Six weeks following the initial GDV the patient developed a sudden onset of gastric bloat. There was no known cause for this but the stomach was successfully decompressed by passing a stomach tube under general anaesthesia. A sample of the gastric contents was sent for analysis which revealed a large growth of candida but no other bacteria were grown (likely from the recent use of antibiotics). The patient was placed on Nystatin (Nystan oral suspension, Bristol-Myers Squibb pharmaceuticals) for 5 days and human probiotics containing lactobacillus (Bio-Kult, Protexin). She showed no other clinical signs and was eating well and behaving normally following the episode of bloat. However sadly she was found unexpectedly dead in her enclosure over 3 months later having shown no clinical signs prior to this. A post mortem examination showed another gastric dilatation volvulus, the stomach having twisted 360 degrees with the gastropexy still in situ. There was also extensive pleural and preicardial effusion in the thorax, likely a secondary heart failure from the GDV.

Conclusion
In the author's experience and in the literature most guinea pigs die prior to apparent clinical signs, at presentation or during initial emergency care of GDVs. One potential reason for survival in this case is the fact that the patient's abdominal muscles were slack from prior breeding. This allowed room for the stomach to twist without substantial pressure being exerted on the greater vessels and diaphragm which would have caused severe shock and mortality quickly. It is also likely due to the quick assessment and surgery by an experienced exotic veterinarian that this patient survived as many general veterinary surgeons are either unaware of this condition or not experienced in dealing with this species. In hindsight the patient could have benefitted from a higher fluid rate during the procedure to aid correction of the hypovolaemia, this may have reduced the hypothermia the patient experienced.
GDVs in guinea pigs are a recognised condition and should be on a differential list when a guinea pig is presented within a veterinary clinic with anorexia, gut stasis and gastric dilatation. Owners need to be made aware of this condition to potentially aid this species receiving prompt emergency care if clinical signs are present. However, more research needs to be done on GDVs in guinea pigs, including post-mortem examinations of those with sudden deaths to help increase knowledge and understanding of this condition.
KEY POINTS
- Gastric dilatation volvulus (GDV) in guinea pigs is a condition which has a historically high mortality rate (75–100%) - often patients are subject to sudden death with little or no signs of illness prior.
- Risk factors which may be associated with development of GDV in guinea pigs could be former pregnancy or currently pregnant, rapid eating, feeding infrequent meals, competition for food, nervous/anxious temperament, stress and increased age.
- Suggested clinical signs of GDV in guinea pigs include weight loss, anorexia, gut stasis, tachycardia, dyspnoea, cyanosis, marked abdominal distention, hypersalivation, abdominal pain, retching, dysphagia, collapse or sudden death.
- Treatment for guinea pigs with GDV is the same as that with dogs suffering from this condition, prompt critical care, treatment of shock and surgical correction is needed for any chance of survival.
- More research needs to be done into this species to understand the aetiology of this condition and disease processes in guinea pigs.


