References
Wound healing for small animal practitioners: a refresher – part one
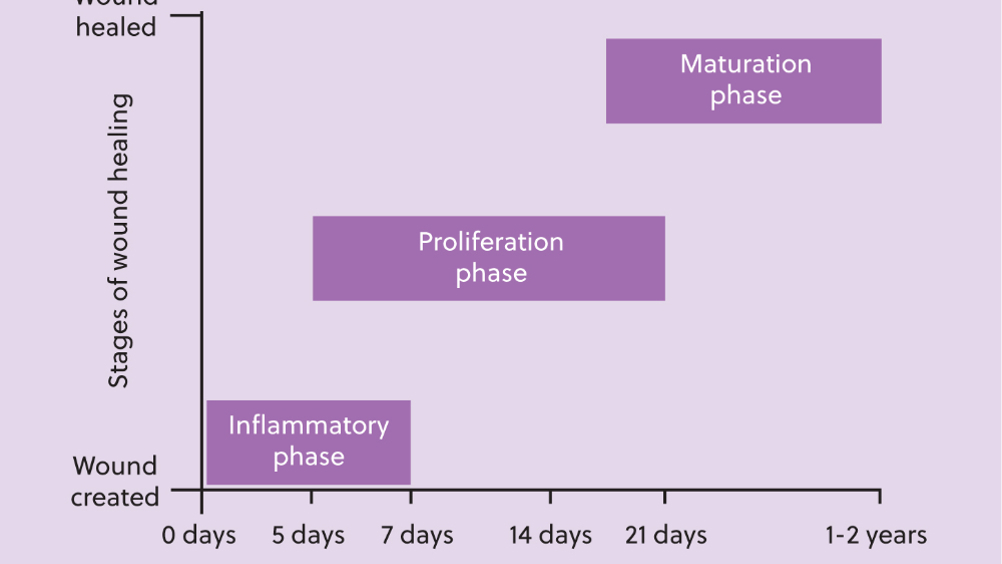
Abstract
Understanding normal anatomy and physiology is essential for veterinary nurses to recognise the pathophysiology of injury and disease. Wound healing is a physiological process that veterinary nurses frequently observe and manage in practice. This article reviews the wound healing process and highlights its clinical relevance throughout. It will be followed by two subsequent articles addressing wound management protocols in dogs, cats and exotic pet species.
In daily practice, whether in first-opinion or specialist referral settings, veterinary nurses encounter patients with wounds almost every day. These may range from uncomplicated surgical wounds to more complex traumatic wounds. A fundamental understanding of wound classification, the physiology of wound healing, and the principles of wound management, is essential for veterinary nurses to deliver high standards of patient care. This article provides an overview of wound healing in commonly encountered species in small animal practice, with useful comparisons between species. It is the first in a three-part series, which will also explore current wound management protocols for small animals and exotic pet species.
Firstly, it is important to define what is meant by a ‘wound.’ A wound is a disruption of the normal continuity of a body structure (Waldron and Zimmerman-Pope, 2003). While a wound is often thought of as a break in the skin surface—true for open wounds—wounds can also be closed, such as haematomas or contusions. Wounds may be superficial, affecting only the top skin layers (eg, a minor abrasion), or deep, penetrating the skin and potentially involving structures like muscle and bone, as seen in deep puncture wounds or degloving injuries.
Register now to continue reading
Thank you for visiting The Veterinary Nurse and reading some of our peer-reviewed content for veterinary professionals. To continue reading this article, please register today.

