Patient details and presenting complaint
Lucy was a 3 year and 2 month old, female neutered Cockapoo. The chief presenting complaint was lethargy and exercise intolerance associated with severe bradycardia at 30 beats per minute (bpm) noted by the referring veterinary surgeon.
General history
Lucy presented to the cardiology department for a consultation. Lucy had been in the owner’s possession since she was 6 months old and was the only pet in the house. She was fully vaccinated and up to date on worming and flea/tick treatment. No coughing or sneezing had been noted. Her urination and defecation were normal, and no vomiting was observed.
Specific history
Approximately 2 days before presentation, Lucy became lethargic and reluctant to exercise. Her appetite was reduced. Her referring veterinarian noted a bradycardia at 30 bpm. No syncopal or collapsing episodes were reported by the owners.
Physical examination
Lucy presented as quiet, alert and responsive with a weight of 13 kg and a body condition score of 6/9. Her mucus membranes were pink and moist with a capillary refill time of less than 2 seconds. Lymph nodes were all within normal limits. The abdomen was not tense or painful and no abnormalities were detected via palpation. Respiratory rate was 32 breaths per minute with normal effort and clear lung sounds were heard on auscultation. Her heart rate was 40 bpm with no murmur present. Lucy’s pulses were strong and no deficits were felt. Her rectal temperature was 37.8°C.
Body systems review of abnormal findings
Bradycardia at 40 bpm, lethargy.
Veterinarian’s differential diagnosis
Lethargy is a non-specific clinical sign. In this case, it was likely the result of the severe bradycardia as, based on the history and physical exam, no orthopaedic or neurological conditions were described or found. Differential diagnoses for bradycardia would include: second degree atrioventricular block, third degree atrioventricular block, atrial standstill, sick sinus syndrome, electrolyte imbalances, myocarditis, hypothyroidism, various toxins and medications (such as beta-blockers or foxglove ingestion). The latter two were thought to be less likely in this case as they would present as acute self-limiting conditions.
Initial diagnostics and results
A full haematology and biochemistry was run by the referring veterinarian, ruling out electrolyte imbalances. Hypothyroidism was felt to be less likely as the thyroxine (T4) and cholesterol levels were within normal reference range. A moderate increase in cardiac troponin I at 0.42 ng/ml (ref <0.05 ng/ml) was suggestive of some myocardial damage, but not myocarditis, and could be caused by the bradycardia.
Blood pressure was measured using Doppler with a size 3 cuff on the tail with a systolic measurement of 200 mHg. Blood pressure measurements were taken following a standardised protocol (Acierno et al, 2018).
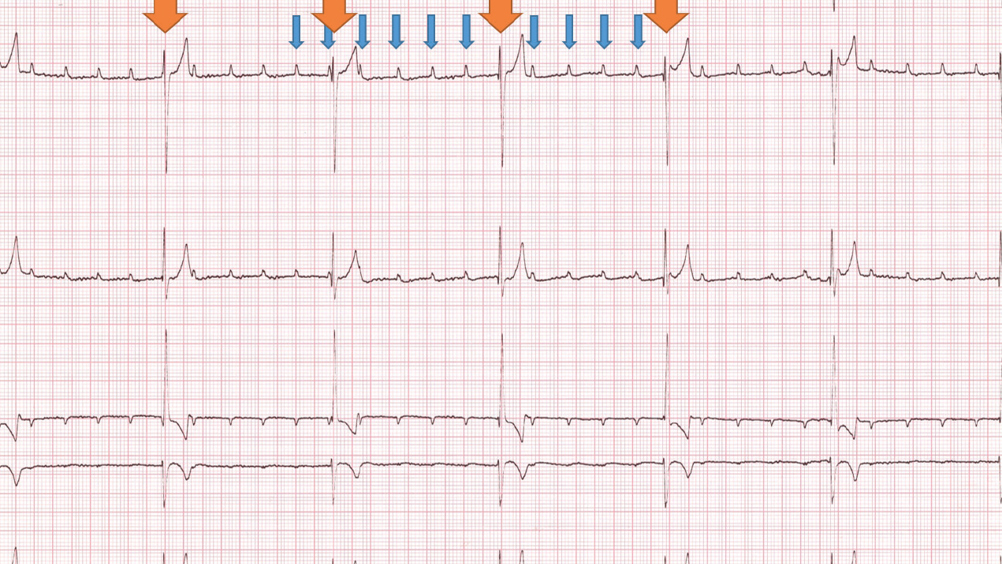
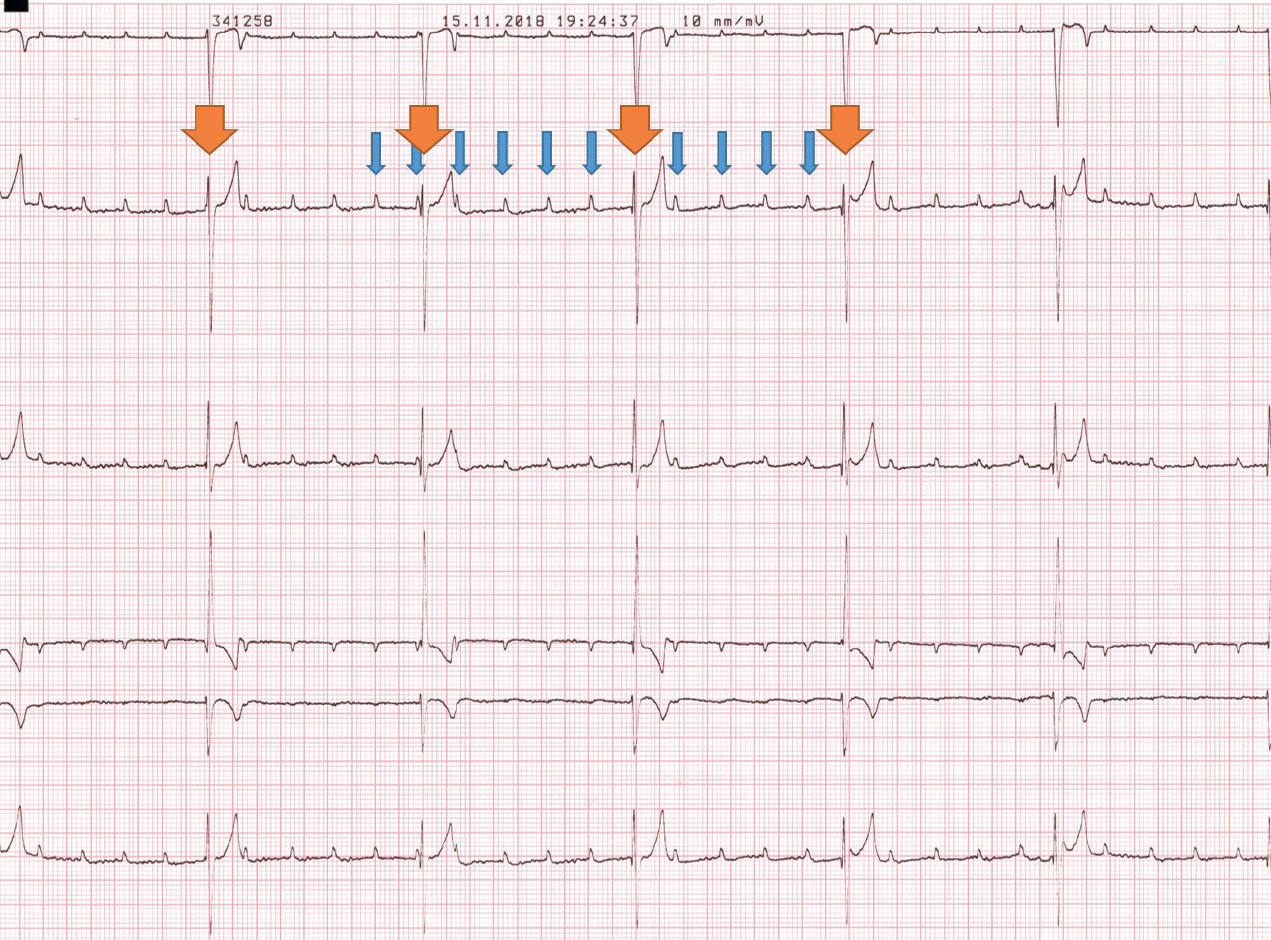
A 6-lead electrocardiogram confirmed third degree atrioventricular block causing bradycardia. A P wave rate of 160 bpm and a ventricular escape rate of 40 bpm were recorded, similar to the findings on physical examination. The P waves were not associated with any of the abnormal QRS complexes and they were marching through the QRS complexes (Figure 1).
Echocardiography was performed to investigate the presence of structural heart disease that could explain the bradycardia. The patient was clipped on the right thorax between the fourth and sixth intercostal spaces from the costochondral junction to the sternum. On the left side, she was clipped between the fourth and seventh intercostal spaces from the costochondral junction to the sternum. The patient was not sedated for this examination.
The patient was placed in right lateral recumbency on a special table with a pre-cut section to allow scanning from underneath the patient. Right-and left-sided echocardiographic views were obtained using cardiac ultrasound. Electrocardiogram monitoring was obtained using electrocardiogram pads attached to three of the limbs (right foreleg, left foreleg and left hindleg).
Echocardiographic examination showed a prominent left atrium with a normal left atrium:aorta ratio of 1.37 (ref <1.5), the left ventricle was slightly rounded in appearance; there were mild mitral and tricuspid insufficiencies although no structural valvular abnormalities were observed. There were no structural abnormalities identified for the right side of the heart. No evidence of masses or other abnormalities in the atrioventricular nodal region were noted. The valvular insufficiencies and left atrial dilation were most likely secondary to the bradycardia.
Diagnosis
The diagnosis was confirmed as third degree atrioventricular block, likely caused by nodal fibrosis.
Treatment
It was decided the best course of treatment was to place a pacemaker to control Lucy’s bradycardia. This was organised for the morning following the examination.
The theatre suite was set up and stocked with interventional equipment for the implantation of a permanent pacemaker (Figure 2). Before induction, Lucy was clipped on the right and left thorax over the heart apex beat, with a large enough area to accommodate the transthoracic pads which were placed and taped on with Elastoplast. The skin was clipped over her right jugular over the midline on her ventral neck down to her manubrium of the sternum up just past the cranial aspect of the scapula, up over the midline on the dorsal neck, down past the base of her ear and jaw. An intravenous catheter (20 G) was placed in the right cephalic vein.
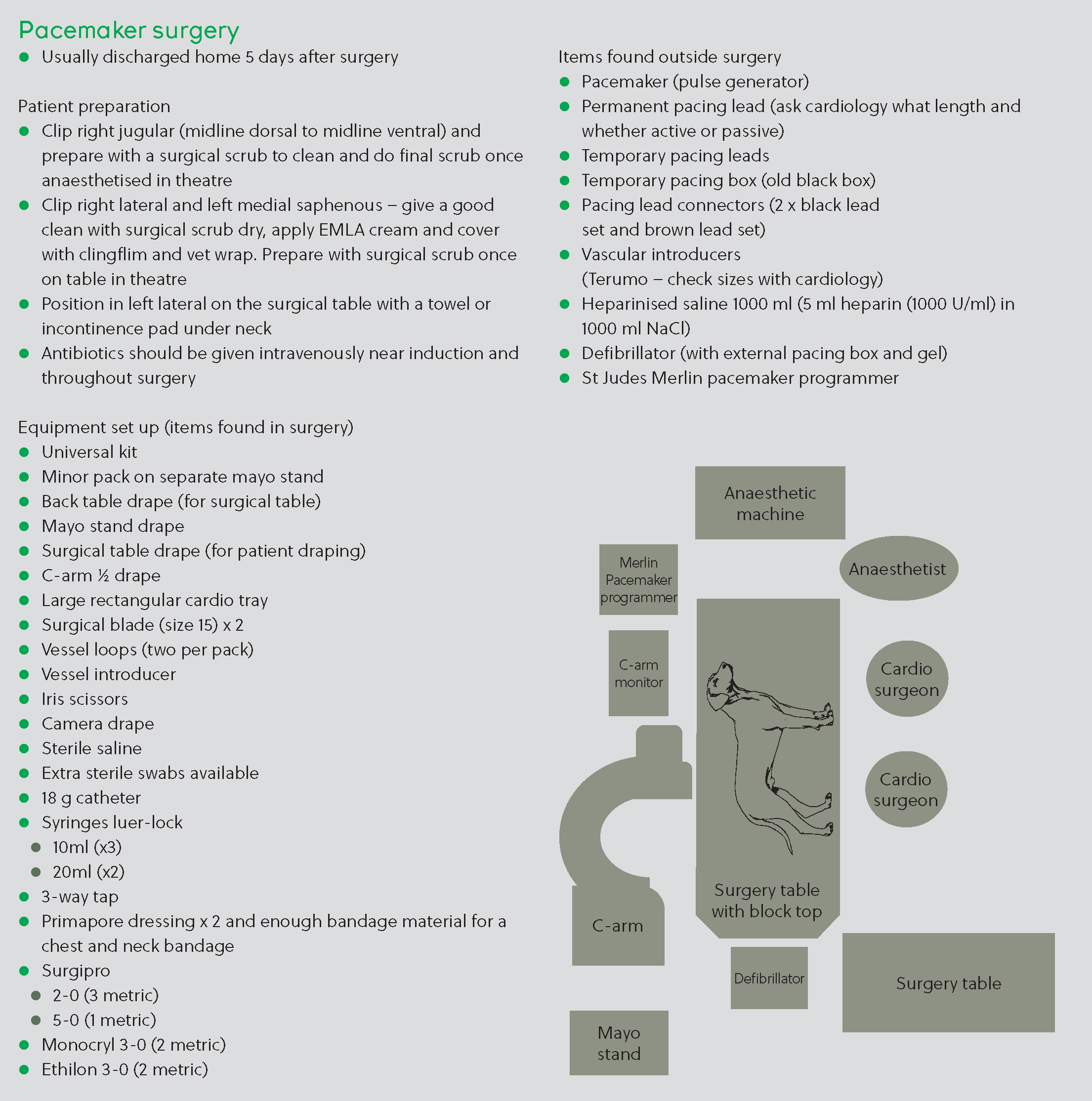
The surgical area was given a good first clean in preparation for surgery using chlorhexidine scrub. Lucy was premedicated with pethidine (91 mg at 7 mg/kg). Lucy’s anaesthesia was induced intravenously with alfaxalone (15 mg at 1.16 mg/kg). The patient was intubated with an 8 mm cuffed endotracheal tube and maintained on oxygen and isoflurane. Following induction, transthoracic pacing was started at 60 bpm with a current output of 50 mA and Lucy was given a skeletal muscle relaxant with atracurium be-silate (3.9 mg at 0.3 mg/kg) to decrease thoracic muscular contractions. The patient was transferred to the ventilator to aid respiration. Lucy was placed in left lateral recumbency, ensuring no rotation of the thorax, and the surgical site was aseptically prepared for surgery with a final skin preparation.
The surgeons were aided in their gloving and gowning. The patient was aseptically draped using a four-quadrant technique and double draping. A cut down over the right jugular was performed, and sutures and vessel loops placed for vein manipulation. Iris scissors were used to make a small incision in the right jugular and a steroid-eluting, active fixation 52 cm permanent pacing lead was introduced through the jugular incision. Fluoroscopic guidance was used for positioning of the lead within the right ventricular apex. The lead was fixed with one nylon ligature at the proximal end. The pulse generator was attached and interrogated with a pacemaker analyser. Results were:
- Lead impedance 850 Ω
- Threshold capture test <0.25 V
- Output 3.5 V at 0.4 msec
- Refractory period 340 msec
- Sensitivity test could not be performed.
The pacemaker was then re-programmed as VVI at 70 bpm (Table 1 gives details of pacemaker coding). Transthoracic pacing was stopped once the pulse generator was attached to the lead. The lead was further secured to the jugular using 3-0 nylon. The position of the lead was rechecked with fluoroscopy. A pocket under the skin in the neck was made for the pulse generator. The pulse generator was detached, the lead tunnelled through the subcutaneous tissue and then re-attached to the pulse generator. The excess lead was gently looped underneath the pulse generator. One fixation suture was placed on the pulse generator to the underlying muscle fascia.
Table 1. Pacemaker pacing and sensing mode coding*
| Position I | Position II | Position III | Position IV |
|---|---|---|---|
| Chamber(s) paced | Chamber(s) sensed | Response to sensing | Rate adaptive |
| O = none | O = none | O = none | O = none |
| A = atrium | A = atrium | I = inhibited | R = rate adaptive |
| V = ventricle | V = ventricle | T = triggered | |
| D = dual | D = dual | D = dual |
*This table demonstrates the coding used for pulse generators (the battery box). The code consists of four letters. The position of the letters informs the technician which chamber is sensed by the pacemaker, where it paces, the response to sensing an intrinsic beat (e.g. inhibiting the pacing), and the response to exercise.
Surgical wounds were closed in three layers with 2-0 poliglycaprone 25 in the subcutaneous tissues and 2-0 nylon in the skin. A full neck and chest bandage was applied and the patient was transferred to the intensive care unit overnight.
Intraoperatively, intravenous cefuroxime 260 mg (20 mg/kg = 3.46 ml at 75 mg/ml) was given every 90 minutes. Postoperatively, two additional intravenous doses were given every 8 hours, followed by oral potentiated amoxicillin 250 mg given twice daily (19.230 mg/kg - 7 days were dispensed). Acepromazine 0.65 mg (0.05 mg/kg = 0.32 ml at 2 mg/ml) and buprenorphine 0.13 mg (10 µg/kg = 0.43 ml at 0.3 mg/ml intravenous) were used for sedation during recovery.
Table 2 shows the post-surgery pacemaker settings.
Table 2. Patient’s pacemaker settings*
| Postoperative | At 1 month follow up | |
|---|---|---|
| Pacing mode | VVI | VVIR for medium activity levels (changed from VVI mode) |
| Base rate (bpm) | 70 | 70 |
| Max rate (bpm) | N/A | 130 |
| Resting rate (bpm) | N/A | 50 |
| Amplitude (V) | 3.5 | 3.0 (reduced) |
| Pulse width (ms) | 0.4 | 0.4 |
| Refractory period (msec) | 340 | 340 |
| Sensitivity (V) | 3.0 | 3.0 |
| Lead impedance (ohms) | 850 | 590 |
| Capture was lost at (V) | <0.25 | 0.75 |
| Sensing was lost at (mV) | Could not be performed | 10.5 |
| Battery life left approx. | >5 years | 8-8.6 years |
| Exercise | Fast reaction time with a slow recovery |
*The pacemaker parameters are reviewed and optimised at each routine follow-up
The following day Lucy was bright, alert and responsive with heart rate 70 bpm, respiratory rate 28 breaths per minute and temperature 37.9°C. She had not required any additional sedation overnight and had not eaten anything. Her bandage was changed and the wounds looked good. The pacemaker was interrogated to check lead impedance, which had reduced to 510 Ω. Over the following 5 days Lucy required sedation for the first 36 hours with buprenorphine (10 mcg/kg at 0.3 mg/ml) 0.43 ml subcutaneously as she was a very calm dog. She was stable, with her heart rate staying at 70 bpm, and preferred a quiet ward to stay in to continue her recovery, which ensured that she stayed calm. She ate intermittently throughout her stay although her owners said she was a very fussy eater at home. Her wounds were healing appropriately. Her bandage was changed every other day and was removed on the day of her discharge with just a light dressing covering the wound.
Outcome
Lucy was discharged 5 days post-surgery. Strict restricted exercise was advised for the first 2 weeks and only short lead walks on a harness for the next 2 weeks. This was to allow the site of the implanted pacemaker lead to heal and avoid pacemaker lead dislodgment. Lucy’s heart rate was set at 70 bpm as this is close to the average resting heart rate for a dog. The pacing mode was VVI, as during home recovery period there was no need to activate the exercise setting.
Revisit 1 month later
On revisit, the owners reported she was a much brighter energetic dog. She was bright, alert and responsive with a weight of 13.6 kg and a body condition score of 6/9. Respiratory rate was 26 breaths per minute with normal effort. Heart rate was 70 bpm with a regular rhythm and matching pulses of good quality. No murmurs were auscultated. No abnormal findings were found in the rest of the body systems.
Her pacemaker was interrogated and was working well. Her settings were changed to allow the rate-responsive setting to be activated (VVIR; Table 1) so Lucy’s heart rate could increase as she gradually increased her level of exercise. The settings are seen in Table 2. Lucy’s exercise was to be slowly increased back to normal over the next 2 weeks.
Discussion
Third degree atrioventricular block is a complete block of any electrical conduction from the atrium through to the ventricles via the atrioventricular node (Kittleson, 1999). Depolarisation of the ventricles is started from an ectopic focus somewhere within the ventricles, at a slower rate than the sinoatrial node or the atrioventricular node, this is called an escape rhythm (Dennis, 2010). The causes of third degree atrioventricular block are still not completely understood, and a definitive cause is unknown, as in this case. It is thought to potentially be a degenerative disease caused by fibrosis of the atrioventricular node, as most cases are in middle- to older-aged patients, or caused by inflammation of the heart wall (e.g. myocarditis, endocarditis), infiltrative (e.g. neoplastic), ischaemic, metabolic (e.g. hyperkalaemia), drug-induced (e.g. digoxin) or traumatic diseases (Tilley and Smith, 2008). In this case, no evidence of myocarditis, endocarditis or structural heart disease was found, therefore atrioventricular node fibrosis was suspected.
Third degree atrioventricular block can be treated in two ways: medical management and surgical intervention (the placement of a pacemaker). Medical management can be attempted using beta-2 agonists (e.g. terbutaline) and methylxanthines (e.g. theophylline), although these are usually not effective. In Lucy’s case her heart rate was too low, suggesting that the origin of the escape rhythm was likely mid-ventricular. Failure of this escape rhythm would result in sudden death, as this is the fail safe. Therefore, medical treatment was not attempted.
Surgical treatment involves the implantation of a permanent cardiac device that will pace the heart at appropriate rates for the individual patient, i.e. a pacemaker. Third degree atrioventricular block is the most common indication for the placement of a pacemaker (Moïse, 1999). Figure 3 illustrates some different stages of the process of pacemaker implantation in dogs. Pacemakers can be placed in two ways, either epicardial pacing or endocardial (transvenous) pacing.

Epicardial pacing is where a specialised lead is sutured onto the outside of the heart on the epicardium connecting to the pulse generator (the pacemaker device). This approach involves a thoracotomy or laparotomy and is not performed as much, as techniques and advances in imaging allow other less invasive surgeries (Kornreich and Moïse, 2014). This route is still used and is more suitable for smaller patients such as cats or ferrets, where transvenous pacing would be difficult because of the size of the veins.
Transvenous permanent pacing is the most common form of cardiac pacing (Kornreich and Moïse, 2014). It is minimally invasive, where the endocardial lead is introduced into the jugular vein and advanced and secured in the right ventricle, with the pulse generator being secured under the skin of the side of the neck. Two forms of endocardial lead pacing are available, single chamber and dual chamber pacing. In dogs, most implantations are of single chamber pacing systems (Estrada, 2015).
Anaesthesia in these cases can be classed as high risk because of the low heart rate and the potential for the escape rhythm to fail. To minimise these complications, temporary pacing can be initiated before induction to aid the anaesthesia until the permanent cardiac pacing has been placed. This is done by transthoracic pacing, placing defibrillation pads on the thorax and transvenous pacing, or by placing a temporary pacing lead into the heart via a peripheral vein (eg lateral saphenous). In Lucy’s case transthoracic pacing was chosen as venous access was more challenging because of the small size of her peripheral veins.
Patients undergoing pacemaker implantation usually show resolution of clinical signs (e.g. syncope, exercise intolerance). In one study, 90% of dogs post-pacemaker implantation had resolution or marked improvement in their clinical signs (Johnson et al, 2007). Another study showed that the mean survival period for dogs post pacemaker implantation was 26 months, with some dogs reaching up to 70 months, and 41% of the study group still being alive at the end of the study (Wess et al, 2006).
Following pacemaker implantation, patients have to return to a practice that can check pacemaker settings. This is non-invasive and uses telemetry to ‘talk’ to the pacemaker (Figure 3d). This allows settings to be optimised for the individual dog and battery life to be monitored. These pacemaker clinics can even be run, as in some practises, by an experienced cardiology nurse, with any changes being checked with the cardiology veterinarian during clinics.
Conclusions
This case report details a patient’s journey, through referral to discharge, for management of symptomatic third degree atrioventricular block. The journey involves assessing that the patient is a good candidate for pacemaker implementation, ensuring a safe anaesthetic procedure, use of specialist equipment to implant the pacemaker, and monitored recovery to decrease risk of postoperative complications. Long-term follow-up visits are required to monitor and optimise pacemaker function. Understanding the steps involved with this type of referral can reassure owners and referring veterinarians and manage expectations.
Key points
- Referral for pacemaker implantation can be a complex and potentially stressful journey, especially if there is not clear understanding of the steps involved.
- The surgical procedure requires specialised equipment and a specialist team.
- The risk during the anaesthetic procedure can be lowered with techniques such as temporary pacing.
- The immediate recovery period is paramount to reduce the risk of complication, such as lead dislodgement.
- Long-term follow-up visits are required to monitor adequate pacemaker function and battery status, but they are minimally invasive.
- Awareness of the patient journey for management of symptomatic third degree atrioventricular block through referral to discharge should reassure owners and referring veterinarians and, overall, contribute to an improved referral experience.