Angiostrongylus vasorum is a nematode parasite that affects the cardiopulmonary system of canids (Elsheikha et al, 2014). It is maintained in a dog-slug-dog transmission cycle. There are many methods by which canine lungworm can be circulated in nature, creating more opportunities for dogs, and wild carnivores, to be exposed to the infective stages. Accidental ingestion of infected gastropods (snails or slugs) harboring infective third-stage larvae, is believed to be the main source of infection. Infection can also be transmitted through ingestion of an infected paratenic (transport) host. This gastropod-borne metastrongyloid parasite, has gained attention from the veterinary community due to its extensive distribution throughout the UK, mainland Europe, Asia and Africa. In the last few years, the number of cases of canine lungworm infection has risen in several regions of North America (Conboy, 2009), with increasing attention being paid to the epidemiology of this parasite in South America (Penagos-Tabares et al, 2018). A similar trend has been also observed in the UK (reviewed in Elsheikha et al, 2014).
Infection with this parasite is potentially life-threatening; and its detection is challenging, due to the wide spectrum of clinical signs and the high frequency of subclinical infection. All ages can be affected, however the most likely age for infection has been reported as around 10 months (Chapman et al, 2004). Over the last few years, there have been advances in the diagnosis and treatment of angiostrongylosis, with several anthelmintic drugs becoming available to help control the disease. In addition, media campaigns have been launched aiming to increase both professional and public awareness of the disease. Used appropriately, anthelmintic products play an important role in the treatment and prevention of lungworm infection. Effective anthelmintics can eliminate adult worms, reduce or stop larval faecal shedding, and reduce the severity of clinical signs and lung damage.
The aim of this article is to emphasise the importance of implementing integrated measures in the management of lungworm disease, particularly in relation to prophylactic therapy using anthelmintics, supportive treatment to reduce health complications, and tailored treatments to address risks associated with A. vasorum infection.
Clinical presentation
Angiostrongylosis is notorious for its non-specific clinical manifestations, occasionally asymptomatic nature, and prolonged course—features that add complexity to the diagnosis of this disease. Dyspnoea and coughing are the most common presenting complaints of cases, seen in both the referral setting (Koch and Willesen, 2009) and in primary care cases (Morgan et al, 2010). These clinical manifestations are not only found in severe cases, but also manifest in milder cases. Coagulopathy and associated haemorrhage, have been among the most serious health consequences of angiostrongylosis. However, these are seen less frequently than the respiratory manifestations (Morgan et al, 2010). Other clinical signs have been also reported such as vomiting, diarrhoea, hypertension, collapse, ophthalmic pathology, cardiac abnormalities (e.g. myocarditis, heart murmurs of grade II to VI, heart failure), and neurological defects that rise from bleeding in or around the central nervous system, secondary to coagulopathies (Søland and Bolt, 1996; Lebon et al, 2016).
Diagnostics
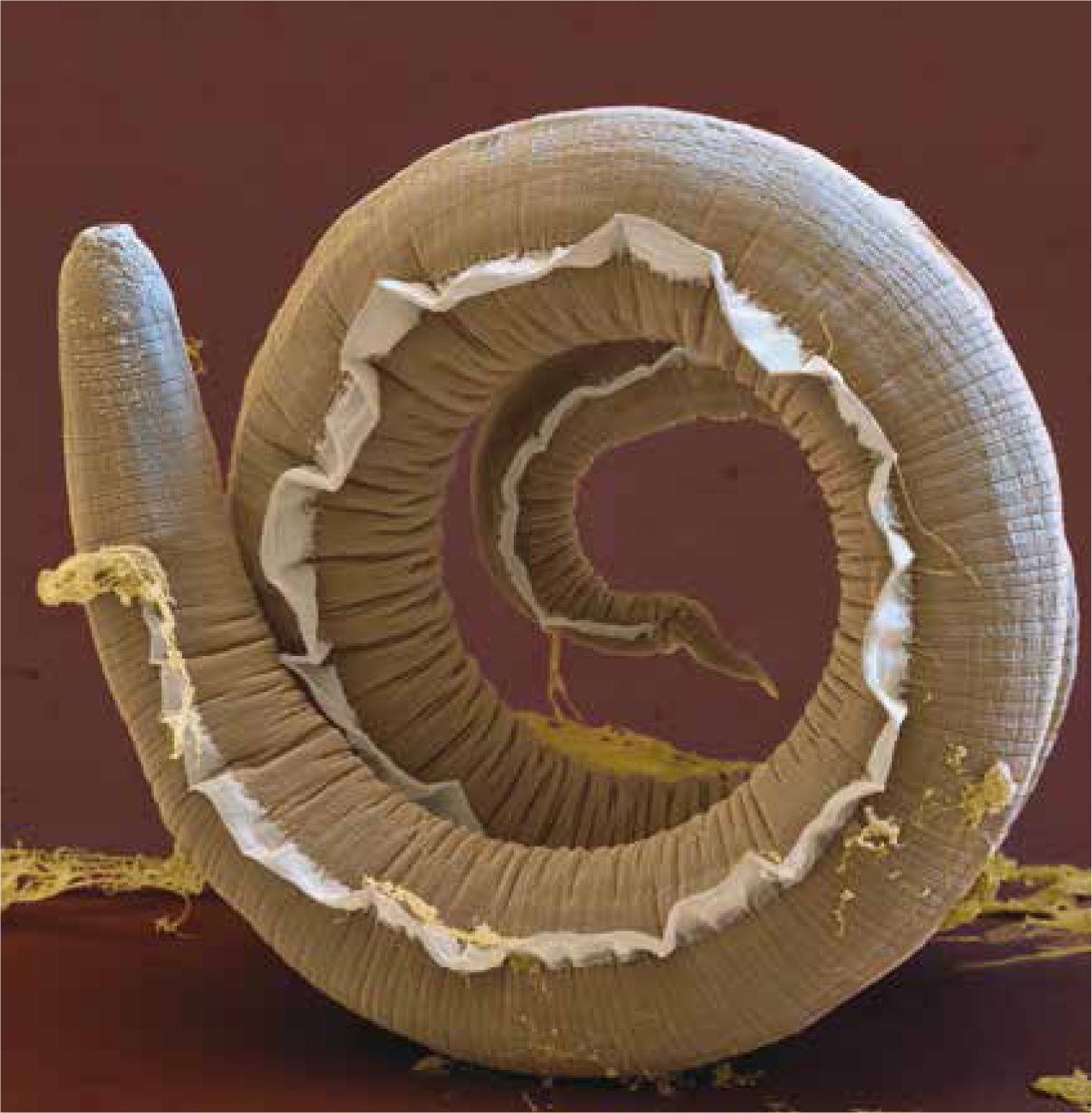
Diagnosis is made by detecting larvae (Figure 1) in faecal samples by the Baermann method (Figure 2). However, the results obtained by this method can be influenced by an inability to detect larvae during the pre-patent period, and by the intermittent shedding of larvae (Elsheikha et al, 2014). This limitation can be mitigated by collecting faecal samples from the same animal over 3 successive days. In response to the obvious need for rapid and easily interpreted diagnostics, a number of polymerase chain reaction (PCR) assays (conventional and real-time) have been developed, in addition to a number of enzyme-linked immunosorbent assays (ELISA) (Elsheikha et al, 2014). One of these methods is the Angio Detect™ (IDEXX Laboratories Inc., Westbrook, Maine, USA) test, which has gained popularity, possibly due to the simplicity and rapidity of obtaining results in approximately 15 minutes. Despite having a specificity of 100% (zero false-positive rate), its sensitivity of 84.6% is lower than that of the external ELISA (94.9%). However, being affordable, fast to run, while maintaining a relatively acceptable level of sensitivity, are advantages that might make Angio Detect™ test a suitable screening tool. The possibility of recording a falsely negative result via Angio Detect™, makes it sensible that veterinarians adopt an alternative testing modality to confirm results—if there is a high suspicion of angiostrongylosis. Larvae can also be detected in bronchoalveolar lavage samples, but this method carries some risks, particularly in dyspnoeic patients.

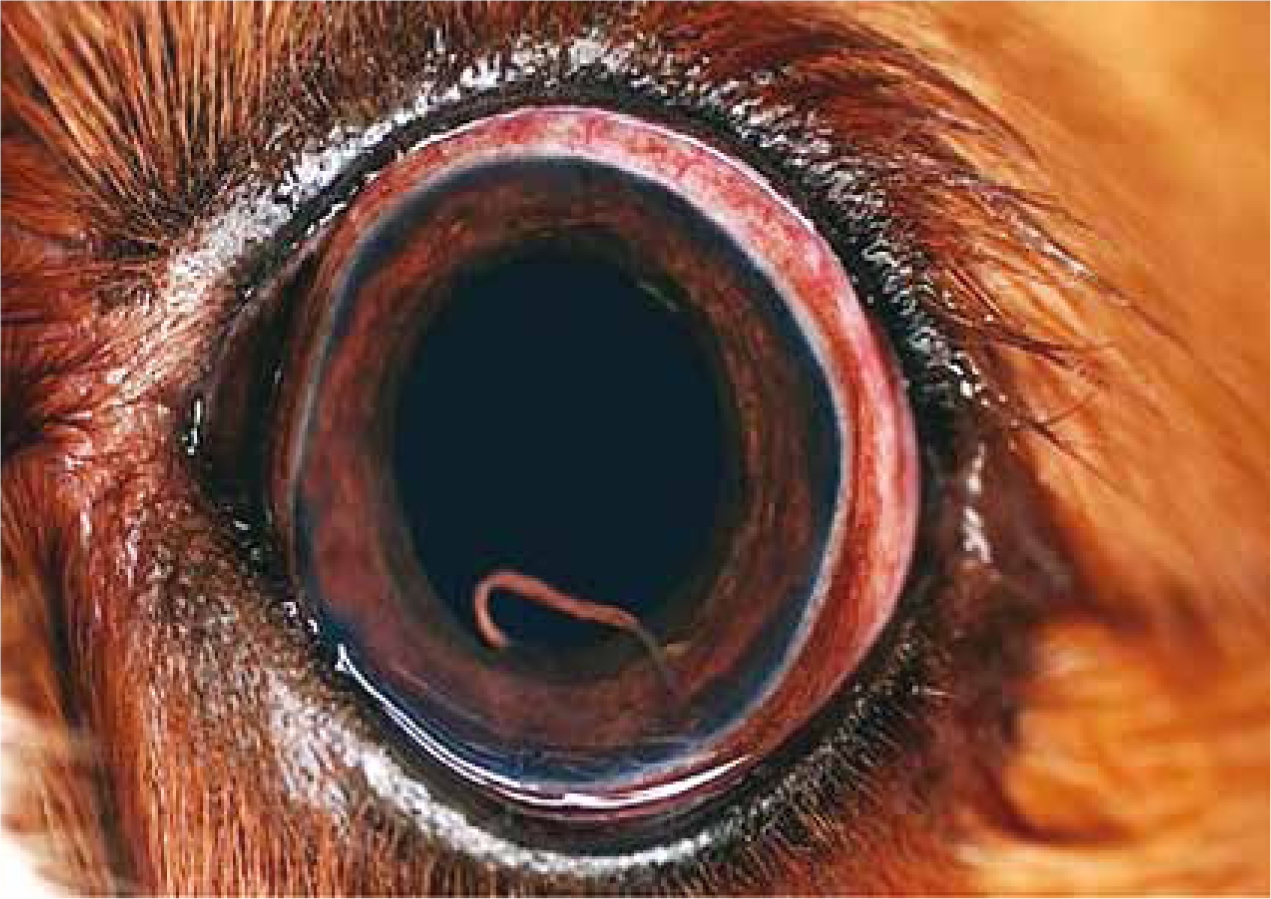
Magnetic resonance imaging (MRI) is the most relevant diagnostic approach when assessing neurological damage, together with cerebrospinal fluid (CSF) analysis. However, this is not advisable in patients suffering from increased intracranial pressure, due to a risk of brain herniation following acquisition of the CSF (Wessmann et al, 2006). Diagnosis of ocular infection by A. vasorum larvae can be achieved by direct visualisation using a pen torch (Figure 3). The larvae can also be aspirated via anterior chamber paracentesis (ACP), for morphological identification to determine the species (Manning, 2007). ACP is likely to occur at a referral practice, due to the highly specialised nature of the procedure. In such cases, faecal examination may be a more cost-effective option for the pet owner. It is important to note, that a negative faecal examination was reported in a dog with an existing ocular A. vasorum infection (Colella et al, 2016).
How well do current anthelmintics work?
In the UK a number of products are licensed to treat angiostrongylosis, and all of these are macrocyclic lactones, moxidectin (a topical form plus imidacloprid; Advocate®, Bayer) or milbemycin oxime in tablet form. In addition, there is increasing interest in the use of ‘off-label’ drugs, such as fenbendazole. Treatment efficacy, for a single dose of the formulation of imidacloprid/moxidectin, was 85.2%, of those dogs still shedding larvae that received a further dose of imidacloprid/moxidectin all animals were found to be Baermann negative (Willesen et al, 2007). While there are additional publications testifying to the efficacy of fenbendazole in naturally occurring canine angiostrongylosis (CA) infections (Brennan et al, 2004; Manning, 2007), in the absence of licensing, the optimal dose, frequency and duration remain unclear. The risks of using such unlicensed products are highlighted by the case of levamisole, which was historically advocated for the treatment of CA due to its potency and rapid onset of action. However, despite having efficacy against CA, this is no longer recommended due to the occurrence of significant side-effects—including anaphylaxis in levamisole-treated patients, thought to result, at least in part, from the rapid increase in circulating worm antigen (Søland and Bolt, 1996).
Besides being licensed for treatment of adult worms, Advocate™ is licensed for monthly use for the prevention of angiostrongylosis and patent infection, with 100% efficacy against L4 larvae and immature adults (L5) of A. vasorum (Schnyder et al, 2009). More recently, it has been demonstrated that a single application prevents any development of immature stages for 4 weeks and avoids lung damage caused by immature adults, based on the persistent efficacy of moxidectin (Böhm et al, 2017).
Milbemycin oxime, commonly available in combination with praziquantel (Milbemax®, Elanco), in a two-dose protocol, achieved a clinical improvement, but without clearance of larval shedding—treatment was extended to 4-weekly treatments to reduce the level of infection, achieving negative faecal Baermann results in 14 out of 16 dogs (Conboy, 2004). Monthly use of milbemycin oxime is indicated for the prevention of angiostrongylosis by reducing levels of infection of immature adult (L5) and adult parasite stages, with a recent study showing a worm count reduction efficacy of 94.9% (Lebon et al, 2016).
Other products containing the same ingredients as Advocate® and Milbemax® are also available. For example: Prinovox® (Virbac) (a POM-V) contains the same combination of imidacloprid and moxidectin as Advocate®; and Milpro® (Virbac), and Milbactor® (Ceva animal Health Ltd) (all POM-V) all contain milbemycin oxime and praziquantel. Milbemycin oxime is also available in combination with spinosad (Trifexis®, Elanco) and afoxolaner (NexGard Spectra®, Merial), both of which have shown to reduce the level of lung pathology associated with angiostrongylosis (Böhm et al, 2014; Lebon et al, 2016).
Organ-specific supportive treatment
Administration of an appropriate anthelmintic has been shown to treat cases of ophthalmic A. vasorum ectopy (Manning, 2007). A more recent study reported that treatment of ocular pathologies requires treatment of the underlying A. vasorum infection, alongside removal of worms present within the eye via ACP (Colella et al, 2016). One of the three clinical cases discussed in this paper, received ocular antibiotics, anti-inflammatory and anti-glaucoma treatments—with the addition of tissue plasminogen activator and adrenaline, to reduce fibrin deposition and haemorrhage while also triggering mydriasis. Although ACP proved effective in these three cases, it is a highly specialised procedure that is rarely performed even at referral level, so its relevance to the treatment of angiostrongylosis at primary care level is limited (Featherstone and Scurrell, 2015).
In addition to providing an effective anthelmintic treatment, supportive therapy in severe cases of anaemia may necessitate a whole blood transfusion, intravenous administration of fluids, or both. While anaemia can be variably present, and may not appear in the vast majority of cases, it seems to be reversible if anthelmintic treatment is given promptly (Brennan et al, 2004; Wessmann et al, 2006; Sasanelli et al, 2008). Treatment for the signs associated with pulmonary hypertension and cardiac failure, includes the use of arteriodilators (e.g. phosphodiesterase-5 inhibitors), ACE inhibitors and diuretics (Brennan et al, 2004; Maltman et al, 2013). A case of persistent syncopal episodes was successfully treated with the bronchodilator theophylline at 20 mg/kg once daily; however, further research is needed to confirm whether this treatment is the most effective, as this was an isolated incident (Brennan et al, 2004). As mentioned earlier, treatment of these secondary clinical signs must accompany anthelmintic treatment of the underlying infection. It is currently thought that the reversibility of these cardiopulmonary signs is based on the severity of infection (Koch and Willesen, 2009), especially as milder cases, seen in first opinion practice, may never display these signs. In one case, a dog was euthanased (14 months after receiving anthelmintic treatment and consequent cessation of larval shedding), due to right-sided cardiac failure, indicating the lasting pathology associated with the original infection (Bourque et al, 2002). In contrast, after receiving treatment for angiostrongylosis and cor pulmonale, another dog showed a decrease in the size of its right atrium and ventricle during follow-up ultrasonography sessions, implying that some clinical signs can be lessened (Brennan et al, 2004).
In dogs with neuropathological complications, anthelmintic therapy should be initiated as soon as possible following diagnosis. The supportive treatment offered to the patient depends on the clinical signs displayed. For example, where the patient exhibits seizures, potassium bromide administration has proven to be an effective management strategy, in conjunction with fenbendazole and prednisolone, to treat the underlying infection (Wessmann et al, 2006). The dog that received this treatment regimen displayed no further seizures, showed no evidence of larval shedding after 11 days, and was considered clinically normal after 3 months. The prognosis for patients displaying neurological defects that received anthelmintic treatment is generally favourable, and complete recovery is possible (Wessmann et al, 2006; Negrin et al, 2008). The lack of treatment could explain the few cases of fatality reported by Denk et al (2009) and Wessmann et al (2006). However, it remains difficult to definitively claim that these patients would have survived, if treated, without knowing all the associated conditions that might offset benefits of the treatment.
Other considerations
Risk-based approach
Regular worming has been an issue of much debate. It is reasonable to use preventative measures including anthelmintics against A. vasorum, particularly in endemic areas. Available evidence indicates, that monthly administration of combination products containing a macrocyclic lactone is effective in the prevention of A. vasorum infection (Willesen et al, 2007; Schnyder et al, 2009; Lebon et al, 2016). However, before prescribing any preventive anthelmintic therapy, a full risk assessment should be performed. It is important to tailor treatment strategies to the prevailing lifestyle, ecological and epidemiological conditions that put the dog at risk of infection. This includes considering the dog's lifestyle (indoor vs. outdoor; hunting vs. non-hunting), travel history (especially to known endemic areas), chance of interaction with wild canids, and frequency of encounters with potentially infected dogs from the same region.
Awareness of risks and signs
Recently, as the veterinary medical and parasitology literature regarding CA has grown, the veterinary community's awareness of this disease has expanded. However, awareness of clinical signs and risk factors of lungworms among dog owners in the general population is still low. CA is often diagnosed at late stages, when prognosis is poor and treatment is difficult; consequently, improving pet owners' awareness of risk factors and clinical presentations might help to reduce delays in diagnosis. To achieve this, it is imperative that veterinary nurses familiarise themselves with the biology, epidemiology and life-threatening clinical manifestations of the often hard to diagnose, sometimes life-threatening disease. Veterinary nurses should be able to provide dog owners with specific information on clinical signs and risk factors in educating them on canine lungworm infection.
Conclusion
Angiostrongylus vasorum has spread from its original southern hotspots, and is now found throughout mainland UK, with cases reported as far north as Scotland. However, this parasite's geographical range has expanded more rapidly than our knowledge of its pathogenicity and evolution. Serious consideration must be given to identifying potential underpinning reasons for parasite expansion. Because effective treatment depends on a timely and correct diagnosis, it is important to use the best available tools to detect A. vasorum infection in order to better guide treatment decisions. Considering the importance of A. vasorum as a cause of potentially lethal infections in dogs, it is recommended that a combination of parasite microscopic detection and molecular diagnostic techniques for early detection, is included in the standard practice for the investigation of lungworm-like illnesses in dogs. In geographical regions with a high prevalence rate of A. vasorum, therapy should be guided by such analysis. Optimal control of lungworm depends, in part, on the use of anthelmintics, but also on better engagement with pet owners, to enhance their awareness and understanding of the disease and of preventive measures. Anthelmintics can disrupt the life-cycle of the parasite, resulting in a reduction in environmental contamination with infective lungworm larvae, and a consequent reduction in the chances of snails and slugs (intermediate hosts) becoming infected. The decision to use an anthelmintic should follow a full risk assessment, which should consider the dog's lifestyle, associated risk factors, and prevalence of the parasite and opportunities that facilitate the acquisition of infection in the local environment. Full recovery of patients with ectopic angiostrongylosis, such as the eye or nervous system, is possible if treatment is initiated promptly. Achieving an optimal strategy of lungworm control depends, besides the use of anthelminics, on a combination of measures that should be utilised coherently, in order to accomplish an integrated management of lungworm infection.

