The kidneys maintain health by regulating water balance, electrolytes, acid base and regulate blood pressure. They also excrete metabolic waste/toxins and play a role in secretion and metabolism of hormones. An acute insult or injury to the kidneys can lead to cellular damage and necrosis. This in turn can result in alteration to the normal functions of the kidney. There can be an abrupt decline in glomerular filtration rate (GFR) characterised by increased serum/plasma creatinine concentration, uraemia (symptoms associated with increased urea and creatinine), and changes in urine output (Legatti et al, 2018) — this is known as acute kidney injury (AKI).
There are four phases of AKI pathophysiology (Monaghan et al, 2012):
- Initiation phase: this starts with the insult/injury to the kidney. At this time the GFR decreases but obvious clinical signs are unlikely to be observed. If the cause can be identified and removed during this stage it may prevent further disease progression.
- Extension phase: during this phase, the kidney insult continues as a result of hypoxia, ischaemia and an ongoing inflammatory response. The GFR continues to decrease as cells undergo apoptosis and necrosis.
- Maintenance phase: the GFR stabilises during this phase as kidney blood flow improves and cellular repair begins. Uraemic complications (vomiting, diarrhoea, changes to drinking and urinating) may become more noticeable; therefore, this is the phase when AKI is generally diagnosed.
- Recovery phase: this is the final stage which can last for months to years. GFR continues to increase due to cellular repair. However, progression to fibrosis might also occur and patients might develop chronic kidney disease (CKD). It is important to avoid further injury/insult during this stage to prevent relapse.
Staging systems
There are numerous classification systems used in the literature for staging patients with AKI including: International Renal Interest Society (IRIS) guidelines (Table 1); Risk, In-jury, Failure, Loss and End-stage renal failure (RIFLE) consensus; Veterinary Acute Kidney Injury (VAKI) criteria and the Acute Kidney Injury Network (AKIN) system (Thoen and Kerl, 2011; Cowgill, 2016). These grading systems use a combination of blood creatinine and urine output (UOP) for staging the disease.
Table 1. IRIS AKI grading criteria
| AKI grade | Blood creatinine | Clinical description |
|---|---|---|
| Grade I | <1.6 mg/dl(<140 μmol/l) | Nonazotaemic AKI:
|
| Grade II | 1.7–2.5 mg/dl(141–220 μmol/l) | Mild AKI:
|
| Grade III | 2.6–5.0 mg/dl(221–439 μmol/l) | Moderate to severe AKI:
|
| Grade IV | 5.1–10.0 mg/dl(440–880 μmol/l) | |
| Grade V | >10.0 mg/dl(>880 μmol/l) |
Volume responsive is an increase in urine production to >1 ml/kg/h over 6 hours; and/or decrease in serum creatinine to baseline over 48 hours.
IRIS, International renal interest society; AKI, acute kidney injury
Common causes
There is a long list of causes of AKI. They can be divided into three categories: pre-renal, renal or post-renal (Herold, 2017). Pre-renal causes include those which affect blood flow to the kidneys such as hypovolaemia, hypotension, congestive heart failure, sepsis or a physical obstruction to the blood flow, such as renal artery thrombosis. Renal causes affect the kidneys directly and can be split into infectious causes (pyelonephritis or leptospirosis) or non-infectious causes (neoplasia, trauma, toxins (i.e. lillies, grapes, non-steroidal anti-inflammatory drugs (NSAIDs)) or glomerular disease). Post renal causes include urinary obstruction due to urolithiasis, strictures or urethral spasm.
History and clinical examination
It is important to obtain a good thorough history in cases where AKI is suspected. This will aid in differentiation of acute versus chronic kidney disease, determine the patient's vaccination status and potential for toxin exposure. Owners may report their pet has been lethargic, anorexic, vomiting, passing diarrhoea and had a change in urine production. Any concurrent health concerns (e.g. cardiac disease) which may increase the risk for AKI or complicate management/nursing care should be identified at this point.
A thorough physical examination is also important so that nurses are aware of the baseline condition of the patient, and any co-morbidities not reported during the history taking can be identified. Patients commonly present with signs of dehydration (dry mucous membranes, prolonged skin tent and possible also sunken eyes) and hypovolaemia (tachycardia, pale mucous membreanes and poor peripheral pulse quality). Therefore, blood pressure should always be assessed and alterations addressed because both hypo and hypertension are risk factors for AKI. Patients might also be mentally depressed, lethargic and weak, and ureamic breath and oral ulceration might also be identified (Mugford et al, 2013). Bradycardia might be present if the patient is hyperkalaemic or in decompensatory shock. Body condition score (BCS) is often normal for a healthy patient, and the kidneys might feel enlarged and be painful for the patient during abdominal palpation. This is in contrast to patients with CKD that might have a poor BCS and small and hard kidney on palpation. If one kidney is larger than the other then this would raise concern for an obstruction affecting the ureter of the larger kidney. The size of the bladder should be determined. A large and painful bladder might indicate a uretheral obstruction, while a small bladder could be as a result of anuria (no urine production), bilateral ureteral obstruction, bladder rupture or the patient having recently voided their bladder. Hypothermia has been documented in some feline cases, however pyrexia may be associated with an infectious cause such as pyelonephritis (Lee et al, 2012).
Diagnosis
The diagnosis of AKI is determined using a combination of the clinical history, physical examination, laboratory findings and diagnostic imaging. Nurses are likely to play an active role in the diagnosis of these patients by performing blood sampling, laboratory work, facilitating imaging, placement of urinary catheters and monitoring urine output.
Blood work
- Haematology: red blood cell (RBC) count is typically within the reference interval but erythrocytosis might be as a result of dehydration/haemoconcentration. Anaemia is often associated with CKD and a lack of eryrthropoetin production, but might occur in patients with AKI due to blood loss (which might have been the primary cause for the AKI) or secondary to gastrointestinal (GI) bleeding associated with uraemic complications.
- Biochemistry: blood creatinine concentration increases with the severity of AKI and is the most commonly used biomarker for GFR (Herold, 2017). Blood urea nitrogen (BUN) increases as the kidney function decreases, however it is also influenced by other factors such as GI bleeding or an increase in production. BUN is therefore considered as a less specific marker of kidney injury compared with creatinine (Cowgill and Langston, 2011). Blood symmetric dimethylarginine (SDMA) concentrations can also be used as a biomarker for GFR, but neither creatinine nor SDMA alone can differentiate between AKI or CKD. Liver enzymes such as alanine transferase (ALT) and alkaline phosphatase (ALKP), and bilirubin might be increased in cases of leptospirosis. Common electrolyte and acid–base abnormalities include hyperphosphataemia, hypocalcaemia, hyperkalaemia and a decrease in serum bicarbonate.
- Additional tests can be submitted to external laboratories and may prove helpful in the diagnosis of specific causes such as leptospirosis tests (microagglutination test, enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR)), or toxin screen tests (ethylene glycol).
Urinalysis
Urine specific gravity (USG) measures the concentration of urine on a refractometer. Typical USGs in healthy hydrated dogs and cats are 1.015–1.045 and 1.035–1.060 respectively (Watson et al, 2015). Highly concentrated urine (>1.045 in dogs and >1.060 in cats) might indicate a pre-renal cause of azotaemia (such as dehydration), whereas isosthenuria (USG 1.008–1.015) in the face of azotaemia suggests there is intrinsic renal disease (Herold, 2017). Glucosuria with normoglycaemia and proteinuria are indicators of tubular damage/dysfunction. A fresh urine sample should be prepared and examined under the microscope to look for evidence of white blood cells, red blood cells (RBCs), and bacteria which may indicate an infection. If these are identified on a free catch sample then a sterile sample should be collected by cystocentesis and sent for bacterial culture and sensitivity testing. It is common to identify granular and hyaline casts if there is active renal tubular damage/necrosis. Approximately 30% of dogs with AKI are reported to have casts on urine sediment examination (Herold, 2017). Struvite and calcium oxalate dihydrate crystals are commonly seen in canine and feline urine samples, however calcium oxalate monohydrate crystals are much less common and associated with ethylene glycol (EG) toxicity (Thrall, 2013).
Imaging
Abdominal radiographs can be used to assess kidney size and investigate for the possibility of urolithiasis (nephroliths, ureteroliths or urethroliths). A nephropylogram can be used to identify ureteric obstructions and retrograde contrast cystograms can be used to detect damage to the lower urinary tract.
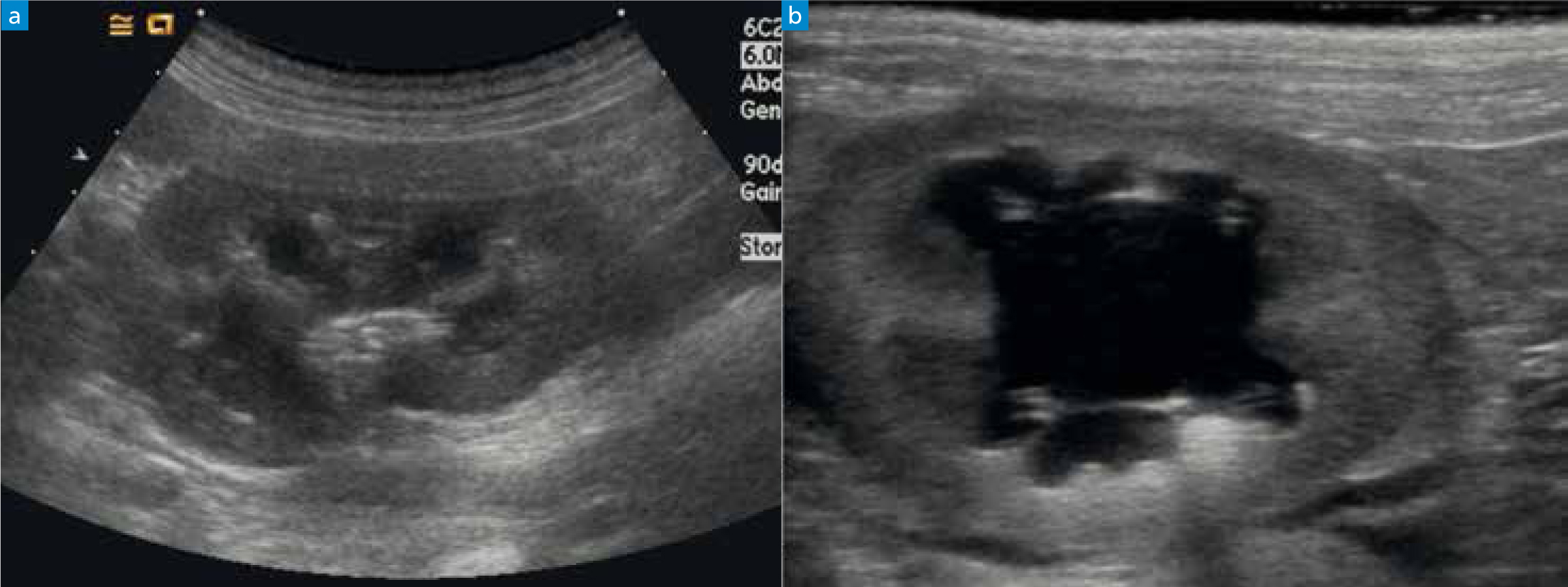
Abdominal ultrasound allows an assessment of the kidney architecture, and urolithiasis might be seen directly or there might be evidence for a ureteric obstruction if there is ureteric and renal pelvic dilatation/hydronephrosis (Figure 1). Ultrasound guided kidney fine needle aspirates or biopsies can be performed but often require sedation +/- anaesthesia and risk damaging the kidney further.
Treatment and nursing considerations
In many cases the underlying cause of the AKI is not identified. Treatment is often supportive and based on management strategies extrapolated from human therapeutics that have largely remained the same for the past 20 years (Cowgill and Langston, 2011). Patients affected by AKI are often hospitalised for days to weeks, and nurses play a vital role in the intensive care of these patients. The nursing team should understand the treatment aims and treatment plan in order to deliver individualised care and maximise the potential for a successful outcome for the patient. Nurses should also be able to identify hospitalised patients with recognised risk factors for AKI in order to avoid AKI occurring. These contributing factors should be minimised/resolved in order to help reduce further damage to the kidneys during this time. In particular, nursing consideration should be made to the areas outlined below.
Alterations in haemodynamics and fluid status
Intravenous fluid therapy (IVFT) is administered in order to maintain perfusion and hydration status. Hypovolaemia and dehydration can exacerbate azotaemia and nurses should monitor for trends in the patient's vital parameters (Table 2). Optimising fluid therapy can be very challenging and the requirement for a particular fluid rate can change quickly. Patients might develop anuria, when fluid overload and congestive heart failure is a risk (Table 3), or patients might develop marked polyuria when very high fluid rates might be needed to maintain normovolaemia and hydration status. Regular bodyweight measurements, UOP and USG can be used as part of the fluid balance assessment of the patient. Vasopressor therapy might also be required to support blood pressure in cases of hypotension refractory to fluid therapy.
Table 2. Clinical signs associated with hypovolaemia and dehydration
| Hypovolaemia | Dehydration |
|---|---|
| Tachycardia | Loss of skin turgor |
| Bounding or weak pulses | Sunken eyes |
| Pale mucous membranes, prolonged capillary refil time | Tacky or dry mucous membranes |
| Hypotension | Positive skin tent |
| Cold extremities | |
| Low rectal temperature |
Table 3. Clinical signs of fluid overload
| Tachypnoea |
| Nasal discharge |
| Hypertension |
| Heart murmur |
| Third spacing — ascites, pleural effusion, peripheral oedema |
| Change in mentation |
| Hypothermia |
Urine production
It is common to see alterations in UOP in patients with AKI and nurses must pay close attention to urine production (Table 4). Ideally a urinary catheter should be placed with a closed collection system to allow accurate quantification of urine production (Figure 2). If a urinary catheter cannot be placed, UOP can be measured by collecting all urine voided on walks/in a litter tray and regular patient body weighing (1 gram weight is equivalent to 1 ml urine). The bladder size should be frequently assessed. A large bladder might be a result of a blockage in the urinary catheter, while a small bladder with reduced UOP would raise concern for oliguria or anuria.
Table 4. Urine output definitions
| Urine output (UOP) | Volume of urine |
|---|---|
| Normal UOP | 1–2 mls/kg/hour |
| Oliguria | <0.5 ml/kg/hour |
| Anuria | None or negligible amount |
UOP (mls/kg/hour) = Volume of urine (mls) / Time (hours) / Patient weight (kg)Positive skin tent
(Langston and Eatroff, 2015)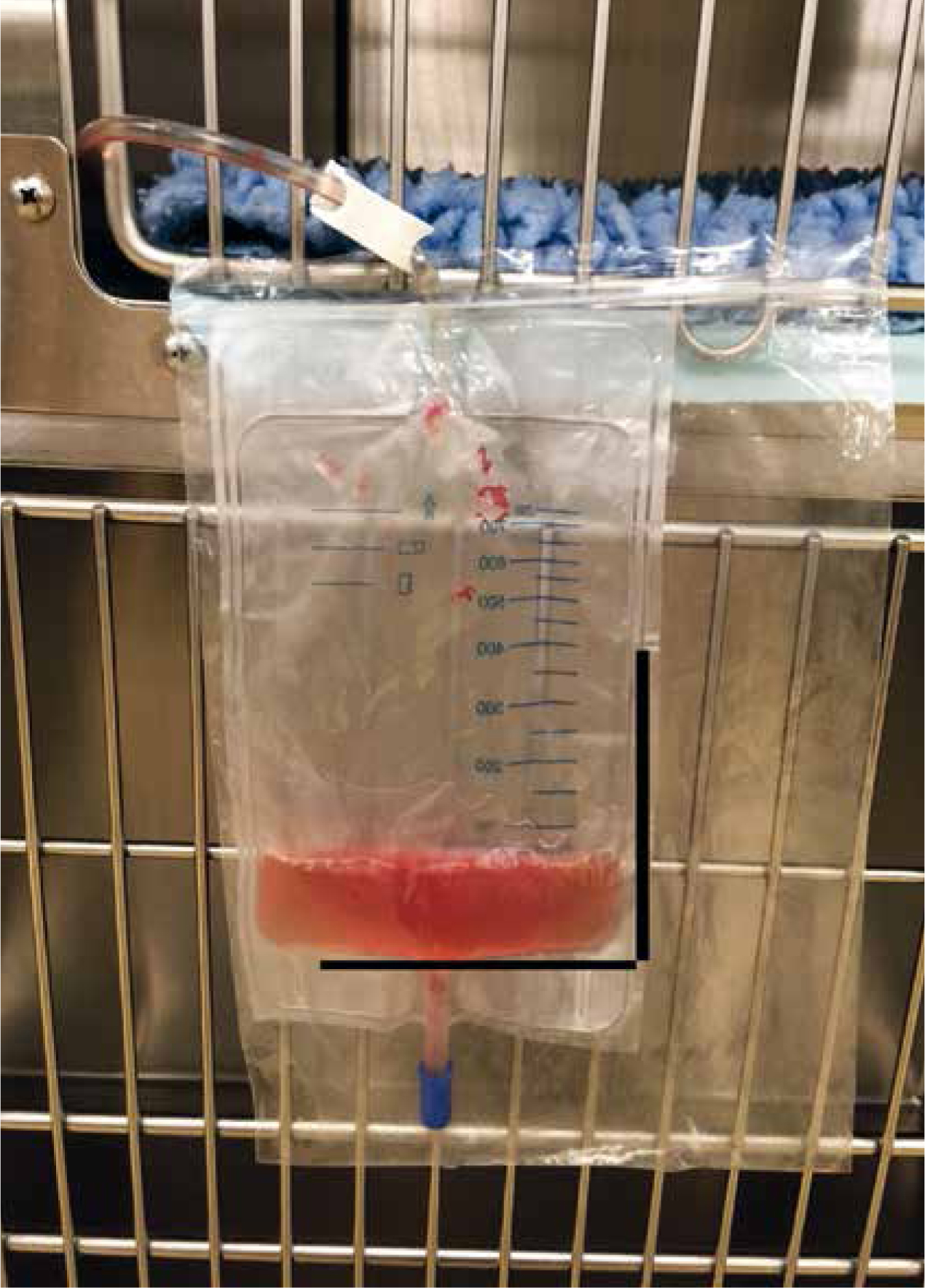
Management of electrolytes and acid–base
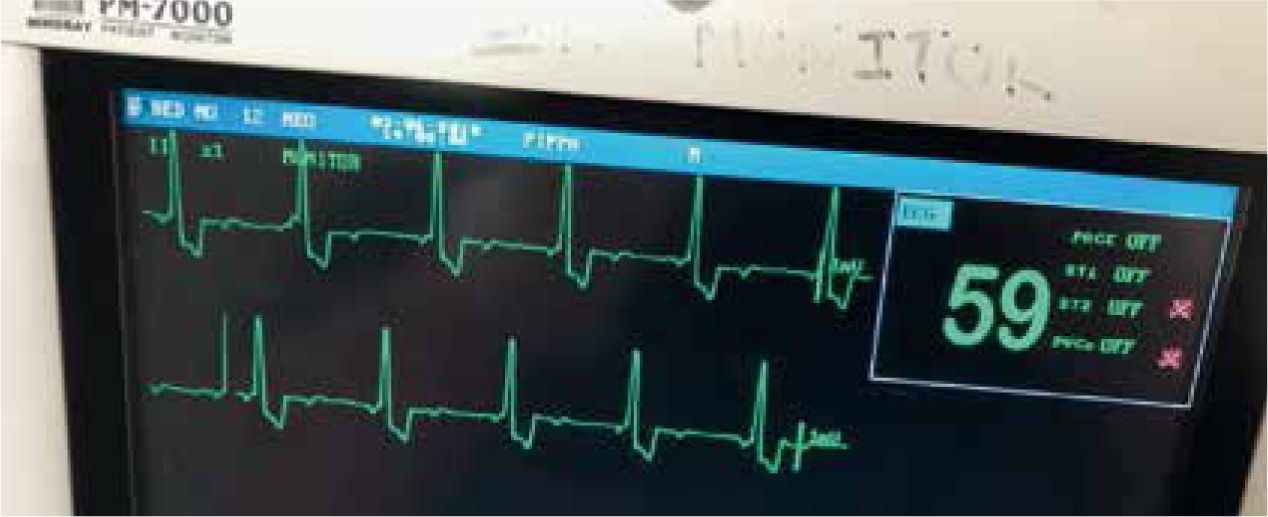
Hyperkalaemia can be a life-threatening complication associated with urethral obstruction or patients that have become anuric because they can no longer excrete potassium. Hyperkalaemia can result in cardiac arrhythmias such as bradycardia, ventricular tachycardia and atrial standstill, and electrocardiogram (ECG) monitoring of these patients is recommended (Figure 3). Hyperkalemia might be managed by intravenous regular insulin and glucose to promote the movement of potassium intracellularly, and calcium gluconate can be administered to reduce the likelihood of cardiac arrhythmias. Further management of hyperkalaemia involves achieving diuresis and establishing urine flow. If the flow of urine can be restored by removing the cause of urinary obstruction or achieving recovery from the AKI event, then some patients can experience hypokalaemia if they develop marked polyuria. Therefore it is important to closely monitor the blood electrolytes in patients during their AKI event and recovery from this phase.
The most common acid–base abnormality documented in these patients is metabolic acidaemia. This occurs as a result of uraemic acidosis and low serum bicarbonate. Fluid therapy alone is usually sufficient to normalise their acid–base status. Sodium bicarbonate administration may be necessary to restore acid–base status if fluid therapy alone is insufficient to normalise their acid–base status. The bicarbonate deficit should be calculated and administered cautiously because rapid administration can cause alterations to electrolytes, induce hypoventilation, hypercapnia and cerebral acidosis (DiBartola, 2012).
Analgesia
Patients might experience pain either due to oral ulceration or kidney and bladder inflammation depending on cause of their AKI. Nurses should be able to perform pain scoring to identify patients that would benefit from analgesia. Opioids are often the first choice analgesic in critical patients because they are often effective, can be given intravenously and are generally well tolerated. NSAIDs should be avoided as they are nephrotoxic and may cause further kidney injury.
Antiemetics and gastroprotectants
It is common for patients to experience nausea +/- vomiting and GI ulceration secondary to their azotaemia. If nurses observe signs such as lipsmaking, hypersalivation, inappetence or anorexia then the case veterinary surgeon should be alerted. Antiemetics, such as maropitant, metoclopramide, ondansetron and mirtazapine, can be administered to alleviate nausea. Faeces should be monitored for signs of GI bleeding. Antacids such omeprazole and sucralfate can help to manage GI ulceration.
Nutrition
Nutrition is always a challenge in the critical patient and provision of nutrition should be established at the earliest opportunity. Nurses play a vital role in ensuring that patients meet their recommended energy requirement (RER). Many patients with AKI will not voluntarily eat and so assisted feeding methods will be required to provide adequate nutrition. Naso-oesophageal feeding tubes work well in these patients as they can be placed without sedation/anaesthesia, can be kept in situ for several days and are generally well tolerated as long as vomiting/regurgitation is managed (Figure 4). Nurses should be able to calculate the patient's RER and devise a suitable nutritional plan, e.g. day one 33% RER, day two 66% RER and day three 100% RER. An ideal diet for AKI patients has yet to be identified, however diets similar to those for CKD patients (moderated protein, potassium and phosphate) or highly digestible bland diets appear logical.
Support for major body systems
- Cardiovascular: cardiac arrhythmias, congestive heart failure (secondary to fluid overload) and abnormal blood pressure readings (hyper and hypotension) can all be complications associated with AKI. Nurses should monitor heart rate and rhythm to identify any abnormalities. ECG monitoring should be implemented in critical patients to observe trends closely and in patients with hyperkalaemia. Blood pressure measurements should be taken at regular intervals so that hypo or hypertension can be addressed promptly.
- Respiratory: nurses should monitor respiratory rate, effort and pattern to identify signs of respiratory distress which could be a result of fluid overload or pulmonary haemorrhage (secondary to leptospirosis). Identification and treatment of the underlying cause is important in addition to oxygen therapy and ongoing respiratory monitoring. Arterial blood gas sampling is the gold standard assessment of patient oxygenation/ventilation. If this is not available then pulse oximetry can be used to assess oxygenation.
- Neurological: nurses should assess mentation every time they interact with the patient. In some cases of severe uraemia, encephalopathy can develop leading to impaired mentation, stupor, tremors and seizure-like activity. Treatment requires supportive therapies to reduce uraemia and manage seizures, and severely affected patients might benefit from haemodialysis.
Management of tubes drains and lines
If patients are requiring hospitalisation for the management of their AKI, they may benefit from the placement of a central venous catheter for concurrent fluid administration, drug administration and frequent blood sampling. Such lines can be placed by the nursing team in either the jugular or saphenous (medial in cats, lateral in dogs) vein and kept in situ for an indefinite time as long as they are appropriately managed and there are no signs of complication (Kirby and Rudloff, 2017).
Patients might also require urinary catheterisation to establish urine flow or to manage ‘ins and outs’. Again, the nursing team should have an understanding of which urinary catheters are appropriate for the patient and how to place them. The clinic should have a standardised operating procedure (SOP) for any device left in situ so the nursing team are aware of how to manage the device and to minimise the risk of complications or hospital acquired infections.
Specific therapies
If the underlying cause can be identified then specific condition-directed therapies should be incorporated into the treatment plan. Treatments might include:
- Antibiotics — administration of the correct antibiotics is imperative in infectious cases of AKI such as pyelonephritis and leptospirosis. Barrier nursing patients with the latter is necessary due to the zoonotic potential of this infection.
- Decontamination — if there has been a known toxin exposure (e.g. lillies, grapes, NSAIDs), decontamination should commence immediately, an antidote or reversal agent sourced if applicable and supportive therapies administered.
- Ethanol — administration of intravenous 20% ethanol can be prescribed to treat ethylene glycol toxicity. Treatment can be administered at 4 hourly intervals for five treatments and then 6 hourly intervals for four treatments. Close monitoring and intensive nursing is required in these patients due to the mentation depression caused by the ethanol administration (Thrall, 2013).
- Haemodyalysis — continuous renal replacement therapy is now a treatment option available in some veterinary institutes (Figure 5). Indications include severe azotaemia, volume overload, anuria, severe acid–base and electrolyte abnormalities or for toxin removal (Welsh, 2017).

Outcomes and prognosis
Survival rates are variable and depend on the underlying cause. The combined mortality rate of AKI in cats and dogs has been reported to be 47%. Non-infectious causes of AKI carry a worse prognosis than infectious causes. Cats have a slightly higher mortality rate than dogs (Legatti et al, 2018). There is no single parameter that has been shown to aid in predicting prognosis, neither urea or creatinine have been shown to be statistically significant prognostic factors (Lee et al, 2012).
The recovery phase can take months and during this time patients are likely to require ongoing medical management and support. In up to 50% of cases the patient will remain azotaemic and progress to a state of CKD (Cowgill and Langston, 2011).
Conclusion
It is important that nurses are able to differentiate between AKI and CKD in order to educate clients, aid in patient work-up and deliver gold standard nursing care. Although AKI carries a guarded prognosis is some patients, in others the disease process can be reversed and patients can go on to have a good quality of life. A prompt diagnosis is key so aggressive medical management can be started with the aim of preventing further damage to the kidneys. These cases often require diligent nursing care in order to support them through the recovery phase.
KEY POINTS
- Acute kidney injury (AKI) is an abrupt decline in kidney filtration rate.
- Causes of AKI can be divided into three categories: pre-renal, renal and post-renal.
- There are a number of grading systems that can be used to stage the patient, which aid in management and prognosis.
- Diagnosis is based on blood work, urinalysis, diagnostic imaging and in some cases specific tests to identify the underlying cause.
- Treatment is usually supportive — if an underlying cause has been identified, such as an infection, then appropriate treatment with antibiotics can be prescribed.
- AKI can be reversible but in up to 50% of cases the patient will remain azotaemic and progress to chronic kidney disease (CKD).


