In terms of anaesthesia, ’neonate’ can be defined as canine or feline patients aged from birth to weaning or <4 weeks of age, and ‘paediatric’ can be defined as those aged 4 weeks to 6 months of age. Paediatric patients present a unique set of challenges and therefore careful anaesthetic planning is required. A variety of factors should be considered because of the anatomical and physiological differences presented in these patients as they are still developing, have a high metabolic rate and limited body energy reserves. Therefore, it is vital that the registered veterinary nurse (RVN) monitoring and maintaining the anaesthetic has a solid understanding of these considerations in addition to the physiological effects of anaesthetic drugs used, and that appropriate monitoring techniques are utilised in order to ensure successful outcomes.
Key physiological differences
Although young patients are capable of rapid repair, it was stated by Rigotti and Brearley (2016) that these patients have a reduced capacity to compensate in response to physiological changes that are caused by the administration of anaesthetic drugs as their organs are still undergoing development, and are particularly vulnerable to lasting damage that may not be identified until later in life.
Cardiovasuclar system
The cardiovascular system of paediatric and neonatal patients is particularly susceptible to the effects of anaesthetic drugs because the myocardium has reduced contractility and the ventricles have a low level of compliance. As a result of this there is a reduced ability to increase stroke volume and cardiac output is therefore reliant on heart rate (Grundy, 2006). In addition to this, young patients possess an immature sympathetic nervous system and therefore the baroreceptor reflex is reduced, and vasomotor control is poor (O'Dwyer, 2017). Consequently, young patients are less able to cope with changes in blood pressure or haemorrhage and it was stated by Rigotti and Brearley (2016) that blood loss volumes as small as 5 ml/kg may result in anaemia and severe hypotension. Therefore, drugs that result in cardiovascular depression or bradycardia should be avoided as this will likely profoundly affect cardiac output and blood pressure. Moon et al (2001) stated that systemic arterial blood pressure in the newborn is significantly lower than in adults and that average systolic arterial blood pressure (SAP) is 54 mmHg, average mean arterial pressure (MAP) is 40 mmHg and average diastolic blood pressure is 30 mmHg. In comparison to this, Haskins (2015) suggested that a MAP above 60 mmHg and SAP above 80 mmHg was required in adults to adequately perfuse vital organs.
Respiratory system
In the paediatric and neonatal patient there are physiological differences in the respiratory system when compared with adult patients. Young patients have a relatively large tongue and a narrow tracheal lumen with flexible tracheal rings and are therefore particularly susceptible to upper airway obstruction. It is advised that paediatric patients are intubated during general anaesthesia, however this can be challenging because of their size. As stated by Dugdale (2010) they also have a high resting respiratory rate because of their increased oxygen requirement compared with adults, potentially resulting in hypoxaemia and rebreathing of carbon dioxide as a consequence. Increasing respiratory rate is the only way that paediatric patients can increase their minute volume as they have a reduced inspiratory reserve volume (Grundy, 2006). In addition to this, the respiratory muscles fatigue quickly because of increased effort and therefore cannot respond to hypoxaemia by increasing minute volume for a prolonged period. Because of a reduced functional residual capacity, patients are particularly prone to atelectasis, therefore, hypoventilation or atelectasis requires prompt identification and intervention through intermittent positive pressure ventilation (IPPV), however this should be used with caution in order to prevent complications such as barotrauma and volutrauma (Day, 2013).
Renal and hepatic systems
Young patients have a variable ability to metabolise drugs hepatically via the P450 enzymatic system (Robertson et al, 2018) resulting in prolonged duration of action and therefore lower doses may be more suitable for these patients and drugs that are reversible may be favourable. There is also an increased risk of hypoglycaemia, because of limited glycogen stores in the liver and poor gluconeogenesis.
Additionally, the renal system has high vascular resistance, resulting in a lower glomerular filtration rate and tubular secretion. Renal blood flow is reduced in neonates, however this has been shown to increase with age (Jose et al, 1971) and because of the immaturity of nephrons the renal capacity to conserve fluids and reabsorb electrolytes (Lúcio et al, 2019) is reduced. This makes young patients particularly susceptible to dehydration and although fluid requirements are higher than in adult patients, they are also at risk of fluid overload with intravenous fluid therapy (IVFT).
Body composition
As stated by Rigotti and Brearly (2016) young animals possess an increased surface area to volume ratio and a higher percentage of total body water in the extracellular space when compared with adults. This gradually decreases over time, and at 6 months of age it is approximately equal to the volume of intracellular fluid. There is also a limited percentage of fat in younger animals, although this does increase with age. Von Dehn (2014) suggested that albumin and plasma concentrations are lower in the neonate than in the adults, and it can take up to 12 months before adult concentrations are reached. Therefore, drugs that are highly protein bound may have a higher likelihood of eliciting a profound response or toxic effects because of an increase in the free, active form of the drug within the circulation. It should also be noted that the blood–brain barrier is not fully established in young patients and is more permeable than in adults (Rigotti and Brearley, 2016).
Pre-operative considerations
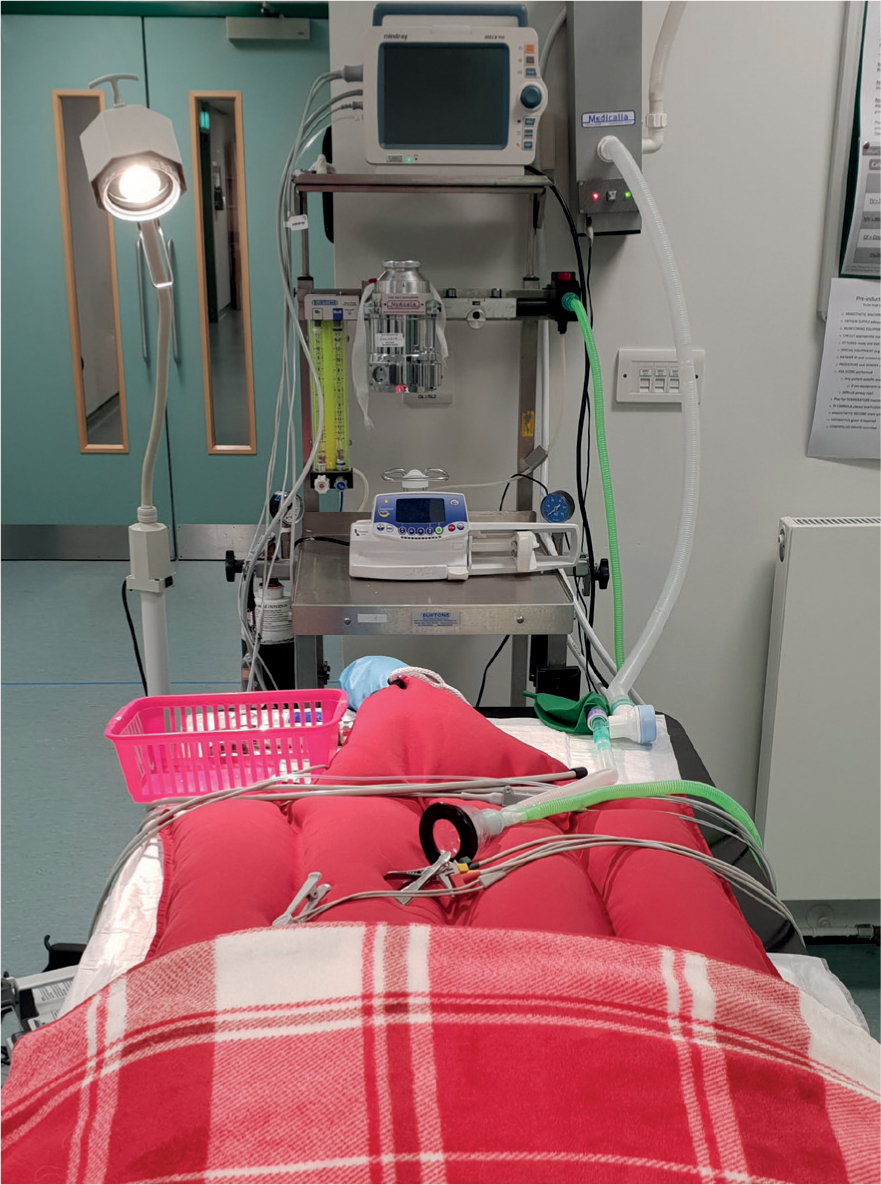
Prior to anaesthesia a thorough physical examination should be performed, however this can be particularly challenging because of the small size and increased respiratory and heart rate of young patients. However, it is recommended that particular attention is paid to the cardiopulmonary system to identify any potential abnormalities prior to anaesthesia. Because of a lower fat content than adult animals, increased surface area to volume ratio and an immature thermoregulatory system, there is a high risk of hypothermia and some anaesthestic drugs also have an effect on the thermoregulatory centre in the hypothalamus and can add to the risk of hypothermia. Root Kustritz (2014) supported the idea that hypothermia can be detrimental to the paediatric patient, therefore every effort should be made to keep patients normothermic in the perioperative period. Prewarming the patient using warming devices is a useful way to prevent hypothermia, however care should be taken to prevent burns (Figure 1).
As previously mentioned, the hepatic and renal systems of the paediatric patient are underdeveloped and therefore careful consideration should be given when using drugs that are metobilised via these routes, as they will often have a profound effect on the patients and a prolonged duration of action (O'Dywer, 2017). As previously mentioned, there is an increased risk of hypoglycaemia so blood glucose (BG) should be monitored regularly throughout the peri-operative period using a glucometer (Figure 2) and glucose supplementation provided if required. The use of a 29G needle of a 100 IU syringe may be particularly beneficial for venipuncture when taking blood samples from neonatal and paediatric patients because of the gauge, and the ability to take small volumes to prevent hypovolaemia, as samples will be taken throughout the peri-operative period. Prolonged periods of starvation are not advised and, although there is an increased risk of regurgitation, hypoglycaemia poses more of a risk to these patients than to adult patients (Rigotti and Brearley, 2016). In patients less than 6 weeks of age, food should not be withheld and in paediatrics over 6 weeks of age, food should be withheld for a maximum of 2 hours (Grubb et al, 2020). To ensure accurate drug dosing an appropriate set of small scales should be used to weigh patients (Figure 3) and, in some cases, drugs may need to be diluted with sterile water or saline (NaCl) for injection because of small volumes, and 100 IU syringes can help accommodate for accurate dosing.
Intravenous (IV) access can be gained through the use of a 26 G to 23 G cannula in paediatric patients, and local anaesthetics creams such as EMLA® cream should be utilised prior to cannulation or blood draws in order to avoid pain and additional stress during the procedure. An occlusive dressing with local anaesthetic cream should be used and left in place for 30 minutes before an attempt to place an IV cannula. Because of the size of neonatal patients, IV access can be difficult to obtain and in some extremely small patients, the intraosseous route via a needle inserted into the medullary cavity of the iliac crest, humerus or femur may be suitable for fluid administration (Mazzaferro, 2009).
Fluid therapy
Young patients have high fluid requirements because of their high percentage of total body water, higher metabolism, increased surface area to volume ratio, immature renal system and reduced ability to concentrate urine. Their increased surface area causes evaporation to occur at an increased rate, contributing to fluid loss, in addition to a reduced ability to conserve fluids, and therefore young patients are at a higher risk of dehydration. Therefore, Lee and Cohn (2017) suggested a maintenance fluid rate of 80–100 ml/kg/24 hour for puppies and 60–80 ml/kg/24 hour for kittens.
Davis et al (2013) and Rigotti and Brearley (2016) suggested that a fluid rate of 3–5 ml/kg/hour should be sufficient in the anaesthetised paediatric patient and this may be increased up to 10 ml/kg/hour if indicated. However, it should be noted that there is currently a lack of evidence-based guidelines for fluid administration during anaesthesia, and the rate suggested is the same as that used in adult cats and dogs. Therefore, it is vitally important that fluid administration is individualised and should be re-evaluated at regular intervals. Fluid therapy should be based on the patient's health status and the procedure being performed to ensure accurate titration in order to avoid fluid overload, as there is a large risk in very small patients because of their body composition (Lopate, 2009). To prevent this from happening a paediatric giving set calibrated at 60 drops/ml should be selected or the use of an accurate fluid pump or syringe driver would be beneficial.
Isotonic crystalloids (e.g. Hartmann's solution) are most commonly used and should be warmed to help prevent hypothermia. An in-line fluid warming device is a reliable technique to use, although Davis et al (2013) suggested that warming fluids may not serve much purpose at low infusion rates as it will not meet or exceed ongoing heat loss in other areas. However, if hypoglycaemia is a concern, and a particularly prolonged length of time under general anaesthesia is anticipated, Rigotti and Brearley (2016) suggested that a 2.5–5% dextrose solution should be administered.
Drug considerations
Premedication
The use of sedatives should be limited in young animals, however the requirement will be dependent on the patient's age, current condition and requirement for IV access. IV cannulation often requires sedation in paediatric patients, therefore an opioid combined with a benzodiazepine may be used to provide sufficient sedation. However, in neonates sedation may not be appropriate, and therefore a mask induction with an inhalant agent may be used and IV access gained as soon as the patient is adequately anaesthetised.
Rigotti and Brearley (2016) suggested opioids have the benefit of providing analgesia and may enhance the sedative effects of a benzodiazepine, although they may produce a vagally mediated bradycardia. In this case, an anticholinergic drug such as glycopyrronium or atropine may be administered, naloxone may also be titrated to antagonise the effects of opioids if undesirable effects such as central nervous system depression, respiratory depression or bradycardia are seen, however this will also negatively affect analgesic properties.
Benzodiazepines such as midazolam or diazepam provide effective sedation and anxiolysis in young animals while having minimal effects on the cardiovascular and respiratory systems.
Although alpha-2 adrenergic agonists such as dexmedetomidine or medetomidine provide reliable, profound sedation and additional analgesia, their use should be avoided in neonatal patients as these drugs result in a profound reflex bradycardia. Grubb et al (2015) stated that cardiac reserve and stroke volume are limited in young patients and therefore cardiac output and blood pressure rely heavily on heart rate. However, in healthy and more developed paediatric patients they may be used with caution and can be reversed with atipamezole at the end of a procedure (Mathews et al, 2014).
Phenothiazines such as acepromazine are also not recommended in neonates as they are not reversible, have a prolonged duration of action, cause profound sedation, and cause vasodilation resulting in heat loss, hypotension and potential cardiovascular collapse (Murrell, 2016). However, Rigotti and Brearley (2016) suggested that in low doses, acepromazine may be used with caution in healthy paediatric patients that are haemodynamically stable.
Analgesia
The provision of appropriate pain management in young patients is critical in successful outcomes and prevents hyperalgesia and the future perception of pain and nociception in the patient. Because of the physiological differences previously mentioned, neonatal and paediatric patients require careful analgesic planning, and nursing care should be altered to meet the needs of these individuals.
Younger animals are known to be more reactive to pain than older animals as they have a lower threshold to noxious stimuli. Therefore, it is crucial that pain is treated promptly and effectively to prevent complex physiological changes to the nervous system, and where possible, pain should always be treated pre-emptively with a multi-modal approach (Mathews, 2014). However, it is important to consider that although acute pain management is vital in a balanced anaesthetic plan, analgesic drugs do come with their own side effects and may result in potential complications. It is for this reason that it is advised to provide multi-modal analgesia to act synergistically on different aspects of the pain pathway, as lower doses can be used and analgesia can be more accurately titrated to effect.
Opioids are a group of drugs that act on μ receptors in the central nervous system (CNS) and affect the pain pathway by reducing the transmission and perception of pain signals (Walsh, 2015). Opioids such as fentanyl, methadone, buprenorphine and butorphanol may be utilised depending on the level of pain that is anticipated with the procedure, however young patients are particularly susceptible to both their analgesic and potential side effects. It was also stated by Mathews et al (2014) that patients over several weeks of age may require adult doses of opioids, although it is still recommended that the lowest end of the dose range or below is used and that the drugs are increased according to effect. Full µ receptor agonist opioids such as fentanyl may be used at low doses and titrated to effect for analgesia in the neonate, however higher doses may be required in patients over 5 weeks of age as shown in a human study by Berde and Sethna (2002), and IV access is required for administration. Methadone is also a full µ receptor agonist, that may be administered both intramuscularly (IM) and IV and may therefore be suitable as part of a pre-medication for a moderately to severely painful procedure, or where intravenous access has not been gained. Similarly to methadone, buprenorphine may be administered IM or IV and has been shown to have minimal respiratory depression in comparison to some of the other opioids, and may be selected for mild to moderate pain.
Non-steroidal anti-inflammatory drugs (NSAIDs) are a commonly used analgesic and are crucial in providing multi-modal analgesia to patients experiencing acute pain, and can provide up to 24 hours of pain relief by acting on the peripheral nervous system. Kerr (2016) highlighted their analgesic properties as they facilitate the inhibition of prostaglandins and reduce the production of peripheral inflammatory mediators. The use of NSAIDs is not recommended in patients under the age of 6 weeks, however, this may differ between drugs and the data sheet should always be consulted before use.
Mathews (2005) suggested that the N-methyl d-aspartate (NMDA) system may be underdeveloped in neonates and therefore NMDA antagonists such as ketamine and the opioid methadone may not be effective on this pathway in these patients.
Local anaesthetics such as lidocaine, bupivicaine or ropivicane may be beneficial in paediatric and neonatal patients, however it is vital that the patient has been accurately weighed prior to administration; 2% lidocaine is most commonly used in young patients in practice, however, it is painful on administration when infiltrated into the tissues because of its acidity. Mathews (2014) suggested that to reduce pain on injection, lidocaine should be injected slowly, and diluted to a 20:1 ratio with 1 mEq/ml sodium bicarbonate to act as a buffer; for example if 2% lidocaine was used, a 2 ml vial could be mixed with 0.1 ml of sodium bicarbonate. Warming the local anaesthetic to approximately 36-37°C can also help reduce pain on administration. Dose rates in neonates are much lower than in the older patients so therefore great care should be taken to avoid overdose.
Induction and maintenance
The induction of anaesthesia may be performed through the use of inhalant or injectable agents. Volatile agents such as isoflurane or sevoflurane may be suitable for mask induction in neonatal patients if intravenous access cannot be gained, and because of its rapid onset, better tolerability, sevoflurane may be more suitable in comparison to isolfurane. Rigotti and Brearley (2016) advised that neonates have a lower minimal alveolar concentration of volatile agents, but this has been shown to increase with age. Once the patient has been induced, venous access should be secured as soon as possible.
If venous access is gained prior to induction, injectable agents such as propofol or alfaxalone (O'Hagan et al 2012a, 2012b) may be suitable for induction. With all injectable induction agents, the drug should be diluted to allow for precise and accurate administration as they are known to cause cardiopulmonary depression and studies such as Zaki et al (2009) suggested that this has been associated with an overall dose reduction.
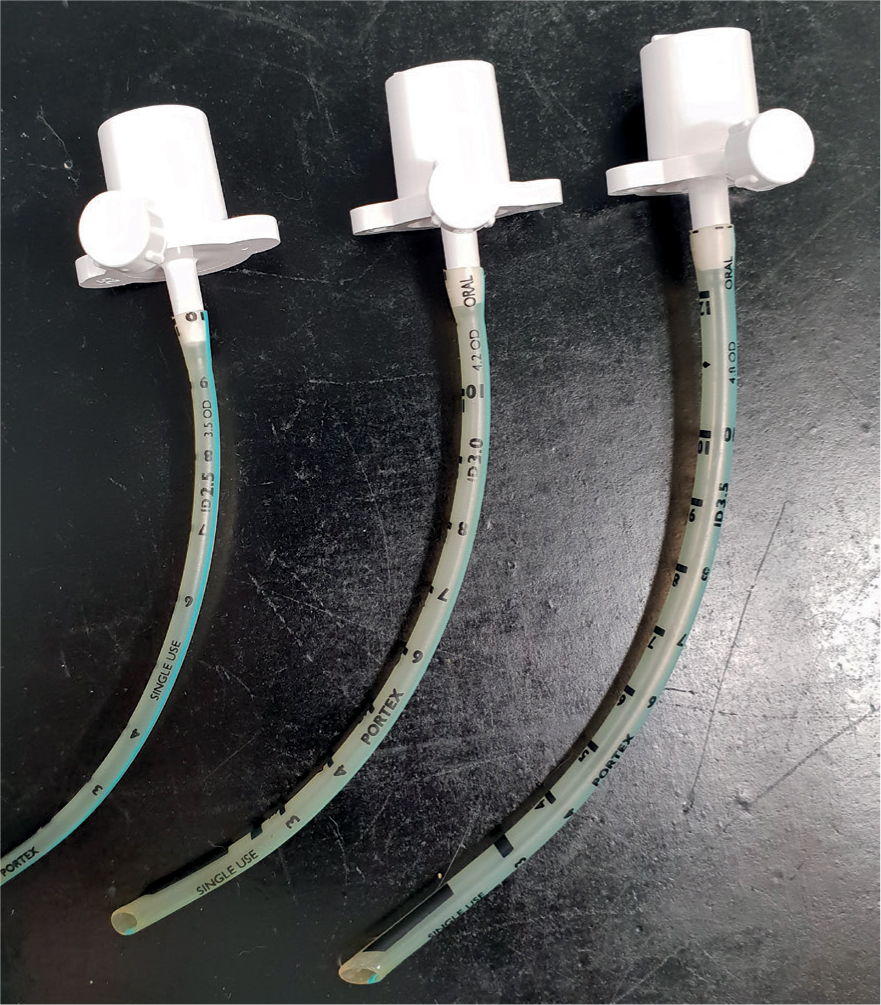
As previously discussed, placing an endotracheal tube (ETT) may be difficult in neonatal patients because of the anatomy of their respiratory system. When placing the ETT, extreme care should be taken to avoid traumatising the soft tissues of the larynx and trachea as this may result in upper airway obstruction or a tracheal tear. If it is not possible to intubate the patient, an appropriately sized facemask should be used. A 2–3 mm ETT may be placed in young animals, and a low dead space adapter (Figure 4) should be utilised to minimise mechanical dead space. When considering breathing circuits for paediatric patients, a non-rebreathing system with low resistance is most suitable such as a T-Piece, Mini-Lack or Bain system as high resistance to breathing is a concern in small patients (Figure 5).

In many veterinary practices, electrical monitoring devices such as an electrocardiography (ECG), pulse oximetry, capnography, blood pressure monitoring (invasive and non-invasive) and a temperature probe are used for monitoring patients under anaesthesia. It should be noted that, although many of these devices can provide extremely valuable information for interpretation, they all have their own limitations and should never replace a highly skilled individual (Grubb et al, 2020). When utilising monitoring equipment a dynamic insight can be gained into different physiological processes happening within the patient's body and can help to alert the anaesthetist to varying trends that may be seen and guide interventions if necessary. Potential disturbances in the paediatric or neonatal patient that may require intervention include but are not limited to: hypotension; hypothermia; hypoxia; and hypoglycaemia (Lopate, 2009).
Recovery
Brodbelt et al (2008) suggested that postoperative deaths accounted for 47% of deaths in dogs and 61% in cats, highlighting the requirement for close monitoring by trained personnel in the postoperative period to reduce the risk of patient mortality. As previously mentioned, BG should be monitored in the peri-operative period including recovery as hypoglycaemia can contribute to a prolonged recovery. Many young patients will require dextrose solution post-operatively because of a prolonged starvation period if supplementation has not already been required, and should be fed as soon as possible following anaesthesia (Root Kustritz, 2014). Pottie et al (2007) recommended that patients should be recovered in a warm environment such as an incubator to prevent hypothermia. Rapid heat loss caused by recovery in an unsuitable environment may result in a life-threatening bradycardia and apnoea that is unresponsive to antichloinergics. Shivering will dramatically increase oxygen consumption and can lead to hypoxaemia if oxygen therapy is not initiated, therefore every effort should be made to maintain normothermia.
Conclusion
Although the RCVS Code of Professional Conduct for Veterinary Surgeons (2012) states that the veterinary surgeon is ultimately in control of the maintenance of anaesthesia, it is imperative that the RVN and veterinary surgeon collaborate and share knowledge to ensure specific patient requirements are met. An understanding of the developmental processes of paediatric and neonatal animals and how they have an impact on an anaesthetic plan is critical; although they share the same fundamental principles it is vital that differences are considered to ensure good patient outcomes. However, there is still a requirement for further research into species specific paediatric and neonatal anaesthesia as most of the published literature is now fairly dated or from human medicine.
KEY POINTS
- Young patients are particularly susceptible to anaesthetic complications such as hypoglycaemia, hypothermia, hypoxaemia and dehydration due to their immature physiology and reduce ability for compensation and therefore anaesthetic management requires careful planning.
- Analgesic plans should aim to be as multi-modal as possible to facilitate a balanced anaesthesia and reduce the risk of potential drug side effects by utilising reduced doses of various drugs that work synergistically.
- The use of anaesthetic drugs that are reversible can be beneficial in young patients.
- Anaesthetic equipment with minimal mechanical dead space and low resistance such a paediatric breathing systems and low space adapters should be used to reduce resistance to breathing and prevent rebreathing of C02 under anaesthesia.


