Canine angiostrongylosis is one of the most important vector-borne parasitic diseases in dogs and is caused by the cardiorespiratory nematode Angiostrongylus vasorum (superfamily: Metastrongyloidea). Dogs become infected after ingesting the third larval stage (L3), usually within an intermediate gastropod host or a paratenic host, such as the common frog (Rana temporaria) or chicken (Gallus gallus domesticus) (Bolt et al, 1993; Mozzer and Lima, 2015). Infected snails, of the species Biomphalaria glabrata, shed L3 into the environment, creating a free-living reservoir of infection (Barçante et al, 2003). Rising numbers of confirmed cases of angiostrongylosis have thrown A. vasorum under the global spotlight, driving research into its epidemiology, diagnosis and risk factors. It is well-established that A. vasorum has spread from its original southern hotspots and can now be found throughout mainland UK, with cases reported as far north as Scotland (Helm et al, 2009). However, this parasite's territory has expanded faster than our knowledge of its epidemiology and control. Therefore, the present review provides the following:
Parasite infection and clinical presentation
A. vasorum has an indirect mollusc-borne life cycle. Dogs become infected when they ingest slugs and snails containing infective third-stage larvae (L3s). Adult female worms lay eggs in the pulmonary capillaries of the dog. The eggs hatch on deposition, and larvae escape into the alveoli and bronchioles from where they are coughed up to be swallowed and passed in the faeces. These first-stage larvae (L1s) (Figure 1) are ingested by slugs and snails (intermediate host) feeding on the dog faeces, and develop to infective L3s within about 2 weeks (Elsheikha et al, 2014).

The A. vasorum incubation period ranges from 28 to 108 days after the dog ingests the L3s. Infection can cause a wide range of clinical signs. Acutely affected dogs may exhibit one or more of the following signs:
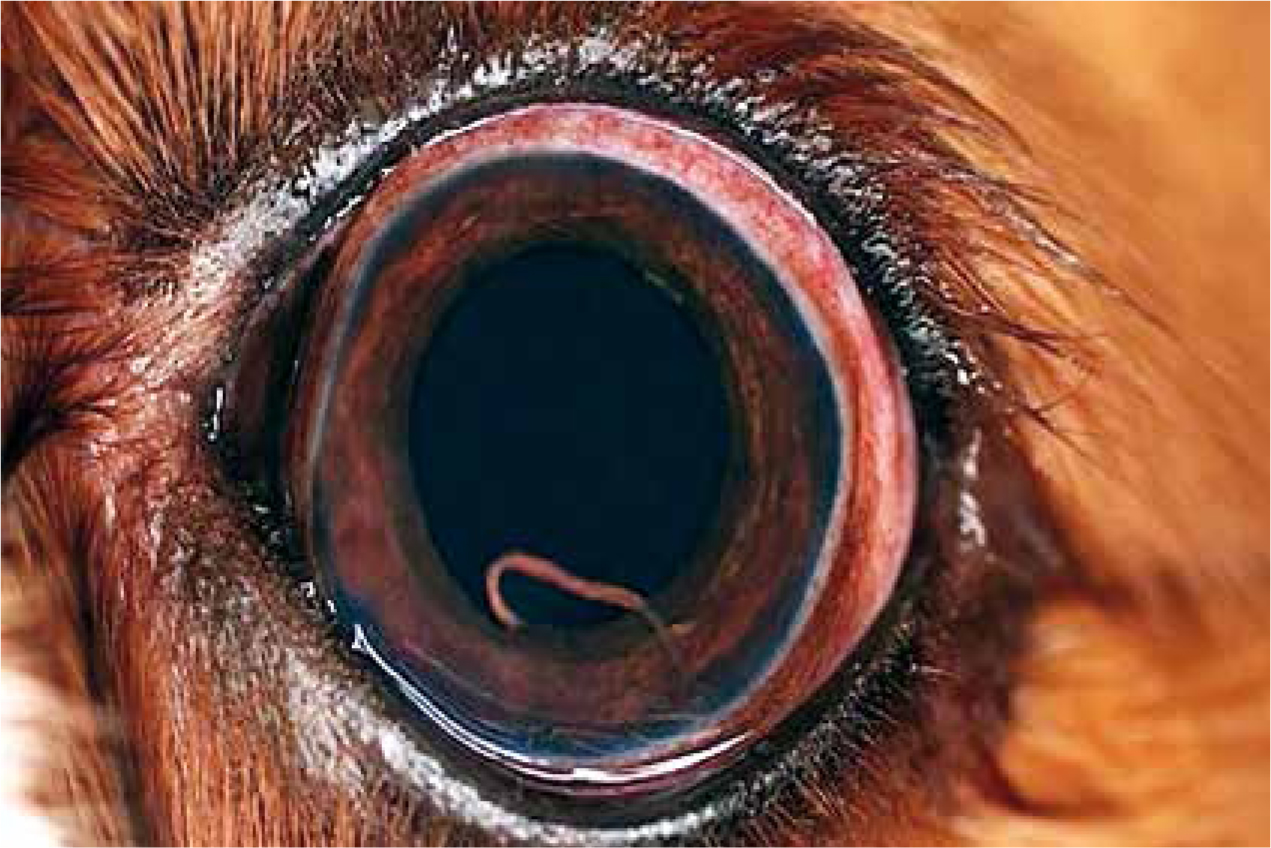
Signs of bleeding disorders, such as haemoptysis, melaena, prolonged bleeding from minor injuries, and occasionally subcutaneous haematomas, are less frequent — but can be fatal (Elsheikha et al, 2014). The molecular mechanisms and host pathways leading to the pathogenesis of an A. vasorum-induced haemoatological disorder are still largely unknown. In severe infections, right-sided heart failure and sudden death may occur. The course of A. vasorum infection is often subtle and chronic; and dogs may show anorexia, weight loss, emaciation, and signs of pulmonary hypertension. Although most pathologies occur in the lungs, larvae and rarely adult worms of A. vasorum can reach ectopic locations such as the brain, bladder, kidney or eye (Figure 2). This complicates the clinical presentation and the clinicopathological picture.
Diagnosis of canine angiostrongylosis
Nucleic acid amplification tests, including conventional polymerase chain reaction (PCR) and real-time PCR, have been developed for the detection of A. vasorum infection in the blood and faeces. A number of enzyme-linked immunosorbent assay (ELISA) assays are also available, including the in-clinic antigen detection kit (IDEXX Angio Detect™), which is considered by some clinicians the first choice test for clinical diagnosis (Elsheikha et al, 2014; Schnyder et al, 2014). The Angio Detect™ test has 100% specificity, but a sensitivity of 84.6%, which is lower than that of the external ELISA (94.9%). Although, the Angio Detect™ test has been considered less suited for use as a screening tool (Schnyder et al, 2014), its convenience and uptake in practice may make it a useful tool to help reduce the clinical uncertainty associated with screening or diagnosis. Baermann technique is another method that can be used for the diagnosis of lungworm by detection of L1s in the faeces (Elsheikha et al, 2014). As a result of the irregular excretion of the larvae, several faecal specimens should be collected over 3 consecutive days. Larvae can also be detected in bronchoalveolar lavage samples, but this method is less sensitive.
While the Baermann method (Figure 3) is vulnerable to human error by overlooking or misidentifying A. vasorum larvae, serological tests are susceptible to the presence of shared antigens or epitopes between different worm species. The risk of cross-reaction was demonstrated when three commercially available test kits for the detection of Dirofilaria immitis gave positive results using sera-containing A. vasorum antigen (Schnyder and Deplazes, 2012). To maximise the reliability of any result obtained, serologic and coproscopic testing should be adopted simultaneously.

Treatment of canine angiostrongylosis
The UK market contains two licensed formulations to treat canine angiostrongylosis, both of which are macrocyclic lactone-based, moxidectin or milbemycin oxime. However, some ‘off-label’ treating also occurs, with fenbendazole in particular.
A topical anthelmintic, containing the active ingredients, imidacloprid and moxidectin, is licensed for the treatment and prevention of angiostrongylosis and available within the UK (Advocate®, Bayer). In addition to being efficacious in the prevention and treatment of angiostrongylosis, dogs treated with imidacloprid/moxidectin demonstrated a significant decrease in lung pathology that was visible using radiography (Willesen et al, 2007); however, these were primary care cases. Schnyder et al (2009) conducted a controlled study consisting of two groups: one treated with Advocate® at 4 days post infection (at which point A. vasorum would be at the fourth larval stage (L4); and the second treated at 32 days post infection (immature adult stage). All dogs showed some lung pathology at necropsy, which was minimal in the group treated 4 days post infection, while multifocal areas of granulomata and thrombi were present throughout the lungs of control dogs.
The damage to lung parenchyma was more severe in dogs treated at 32 days post infection than it was in those treated at 4 days post infection, indicating a window for A. vasorum to cause pathology during its pre-patent period. Another common licensed broad-spectrum de-worming treatment in the UK is Milbemax® (Elanco) — a chewable tablet containing milbemycin oxime and praziquantel. Milbemax® can be given safely to pregnant bitches and during lactation (Rinaldi et al, 2015).
Other products containing the same ingredients as Advocate® and Milbemax® are also available. For example, Prinovox®, POM-V, contains the same combination of imidacloprid and moxidectin as Advocate®; and Milpro® and Milquantel®, both POM-V, and contain milbemycin oxime and praziquantel (as does Milbemax®). Milbemycin oxime is also available in combination with spinosad (Trifexis®, Elanco) and afoxolaner (NexGard Spectra®, Merial); both of which have been shown to reduce the level of lung pathology associated with angiostrongylosis (Böhm et al, 2014; Lebon et al, 2016). However, Trifexis® is unlicensed for treatment and prevention of lungworms, while NexGard Spectra® is licensed to prevent lungworm in dogs.
Fenbendazole has been used to treat A. vasorum infection ‘off-label’ for many years, at varying dosages (25–50 mg/kg) and frequencies (~5–21 days). This may be owing to the belief that a gradual killing of worms, as is accomplished by the extended treatment period, will decrease the risk of anaphylaxis (Bolt et al, 1993). However, at least one report of anaphylaxis exists that could be attributed to fenbendazole administration (Wessmann et al, 2006). Panacur® (MSD Animal Health) is a fenbendazole-based anthelmintic, which is common in veterinary practice and can be used in pregnant bitches (Epe, 2009). Although many case reports have shown fenbendazole to be effective in treating angiostrongylosis (Brennan et al, 2004; Chapman et al, 2004; Manning, 2007), a thorough clinical trial is needed before advice can be given on dosage and frequency.
Prevention of canine angiostrongylosis
Over the last few years, small animal clinicians and some specialist parasitologists have been striving to define an efficient strategy for prevention of canine angiostrongylosis. Despite considerable efforts, there is a lack of standardised guidelines for the prevention of this disease. There is also variation in preventative strategies from practice to practice, suggesting that an evidence-based assessment is needed to determine the most efficacious approach. Currently, veterinary professionals use preventative measures including anthelmintics against lungworms, particularly in endemic localities. Scientific evidence indicates that combination products containing a macrocyclic lactone (e.g. moxidectin or milbemycin oxime), administered monthly, are efficacious as preventatives for canine angiostrongylosis. However, prior to prescribing any preventative drug, full risk assessment of the lungworm present or expected to be present should be performed. Knowledge of the animal's lifestyle and care regimen is essential for predicting the risk of lungworm infection, and this should be taken into account when designing a deworming protocol.
Other canine lungworms
Other, less common, nematode species can affect the upper and/or lower part of the respiratory tract in dogs and cats. Dogs can be exposed to these respiratory worms during travel to areas where these parasites are endemic. Crenosoma vulpis is a lungworm of foxes, dogs and badgers across Europe and North America, and has been reported in a domestic dog in the UK (Cobb and Fisher, 1992). Adult worms live in the trachea, bronchi and bronchioles, and cause coughing, sneezing, and inappetance. The parasite life cycle is indirect and its transmission requires the ingestion of molluscs as intermediate hosts. Diagnosis is based on detecting larvae in faecal samples using the Baermann method or faecal flotation techniques.
Eucoleus (Capillaria) aerophila (C. aerophilia) is another lungworm that inhabits the trachea, bronchi and bronchioles of dogs, cats, foxes, and other carnivores (Conboy, 2009). Infection is usually asymptomatic in dogs and cats, apart from a slight cough, and occurs when the animal ingests an infective egg or an earthworm (paratenic host) containing infective larvae in its tissues. The infective larvae leave the eggs or earthworm in the small intestine of the definitive host, penetrate the intestinal wall, and are carried in the blood to the lungs where they develop to the adult worms in the tracheal and bronchial mucosa. Diagnosis is via visual detection, using faecal flotation or tracheal wash of eggs with characteristic bipolar plugs and a rough ‘netted’ surface.
Eucoleus (Capillaria) boehmi is called the ‘nasal worm’ of dogs and foxes. This parasite infects the mucosa of the nasal cavity and the frontal and paranasal sinuses, and some infected animals may exhibit sneezing and mucopurulent discharge (Hodžic et al, 2016). The life cycle of this parasite is similar to that of C. aerophilia. The diagnostic stage, again detected by faecal flotation or nasal wash, is the egg with unique bipolar plugs and rough ‘pitted’ surface.
Filaroides milksi and F. hirthi both live in the terminal airways, bronchioles and alveoli in dogs. Infection is usually asymptomatic, although coughing and dyspnoea may occur, and miliary nodules may be seen at necropsy. The life cycle is direct and infection occurs via ingestion of larvae. Transmission occurs easily within kennels, because the larvae are infectious when passed in faeces. Diagnosis is based on the detection of larvae or eggs in faeces, or in airway cytology specimens. Radiographic examination may show a diffuse interstitial or focal nodular pattern (Conboy, 2009).
Conclusions
Scientific literature and case reports pertaining to A. vasorum infection stretch back over 30 years, suggesting that lungworm is not an entirely new threat. Indeed, the first UK case was confirmed by Jacobs and Prole in 1975. However, there appears to have been a lag in the time taken for the veterinary profession to respond and begin trying to understand the epidemiology of angiostrongylosis. As the territory inhabited by A. vasorum continues to expand, more research is needed to better equip the veterinary professional to combat this spread, and minimise the risk of angiostrongylosis in domestic dogs. In order to better identify dogs suffering from angiostrongylosis, surveys of clinicians are required to compile confirmed cases and help detect any patterns in clinical signs associated with this disease. In addition, which products clinicians are actually using to treat cases of angiostrongylosis remains unknown. Assessment of treatment trends across the UK, along with the efficacy reported by clinicians using current anthelmintics, is needed in order to prevent and treat A. vasorum effectively, and highlight where this is not the case. A drug trial comparing the efficacy of moxidectin, milbemycin oxime and fenbendazole for the treatment of dogs naturally-infected with A. vasorum would be of great value to the field of small animal practice. Expanding present knowledge of the importance of comprehensive risk assessments and evidence-informed interventions could be particularly important in arriving at evidence-based decisions, avoiding unnecessary treatment, and allowing veterinarians to offer informed counselling to pet owners.

