Care must be taken when describing Angiostrongylus vasorum infections as ‘lungworm’, since other nematode species, especially Crenosoma vulpis, are also causes of respiratory disease in dogs in the UK (McGarry and Morgan, 2009). The life cycle of A. vasorum is well established (Bolt et al, 1994). Important points to note are that first stage larvae (L1) (Figure 1) pass out in the faeces, and develop further in gastropod mollusc (slug and snail) intermediate hosts, with infection occurring when dogs ingest infective third stage larvae (L3). This is most likely to occur through intentional or accidental consumption of infected slugs or snails (Morgan et al, 2005). Infection in dogs most often causes mild to moderate respiratory disease, though a wide range of presenting signs is possible and disease can be severe and sometimes fatal (Koch and Willesen, 2009). These other signs are largely caused by bleeding disorders, the mechanism of which is unclear, and can result in neurological, gastrointestinal, ocular, dermatological or other disease depending on the site of the bleeding. Persistent bleeding after injury or surgery can occur. Other signs such as syncope, lethargy, and stunted growth are probably attributable to lung pathology. Treatment of angiostrongylosis focuses on killing the worms, and this can be achieved by administration of one of a range of anthelmintic drugs, as discussed below. Supportive treatment is recommended depending on the clinical signs (Koch and Willesen 2009; Helm et al, 2010).
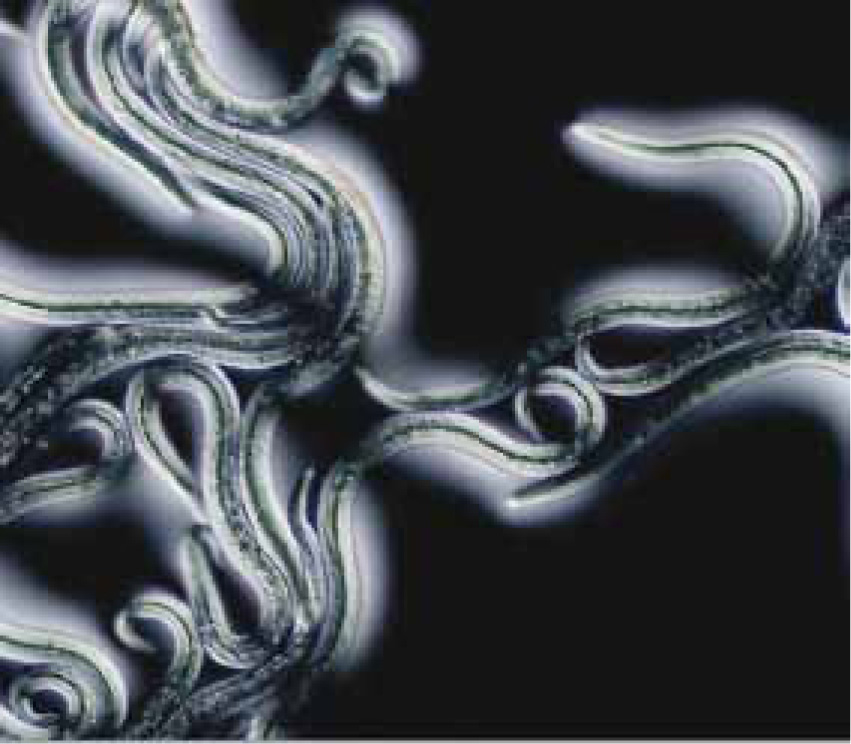
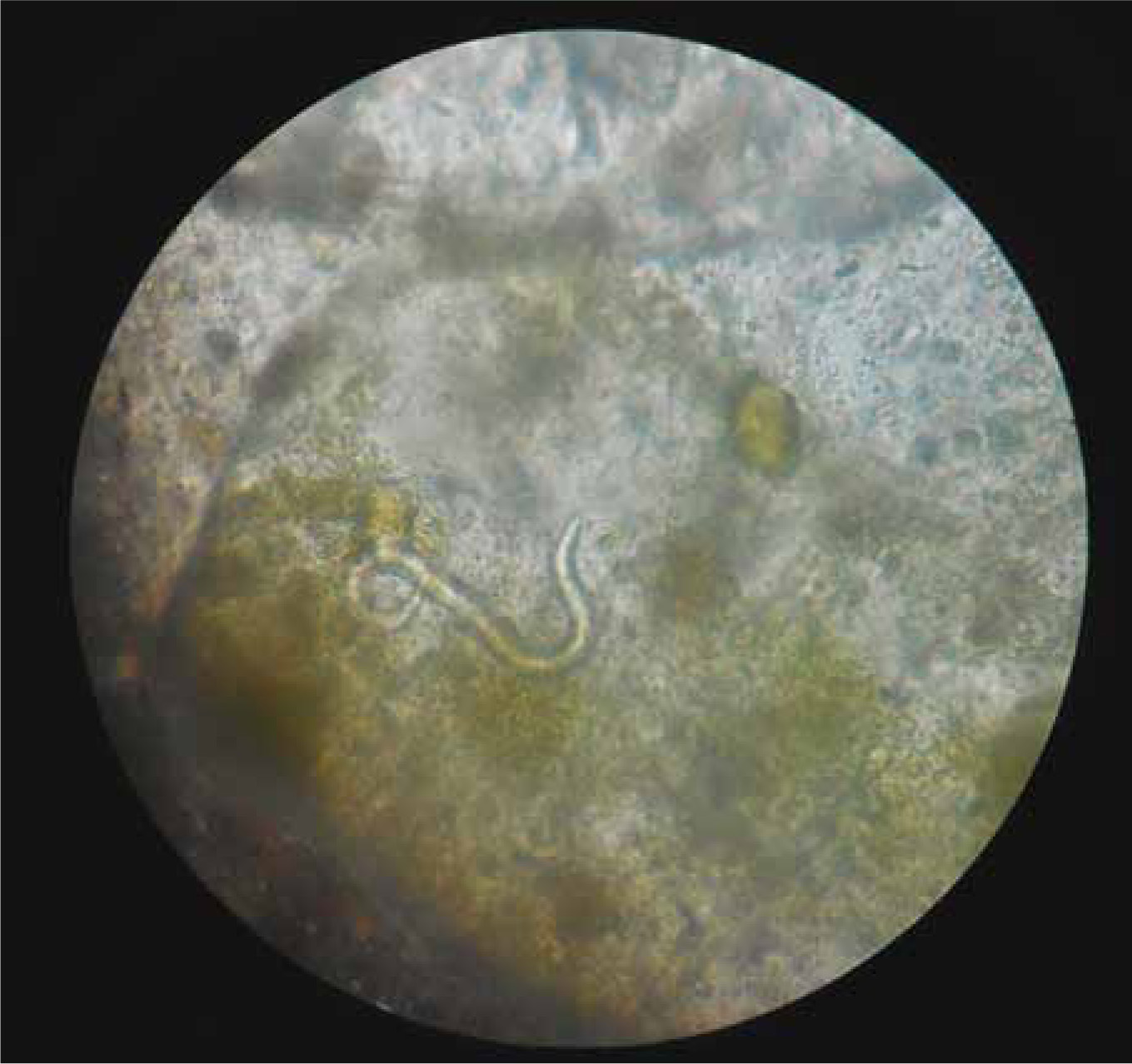
A major challenge in clinical management of angiostrongylosis is diagnosing infection in the first place (Traversa and Guglielmini, 2008). This has traditionally been achieved by recovering and identifying L1 larvae in the faeces, using a modification of the Baermann technique. Faeces (ideally 10 g or more) are placed in a muslin pouch and submerged in water, such that the larvae actively migrate out of the faeces and sink to the bottom of the container. If a tapered container is used (e.g. an inverse conical beaker or wine glass), larvae concentrate at the bottom, and can be recovered by pipette and placed in a cavity microscope slide. Larvae are very active and in clinical cases are usually present in large numbers. After killing by adding a drop of Lugol's iodine, they can be examined under high power for specific diagnosis. L1 of A vasorum have characteristic tail morphology, with a sinusoidal kink and notch that distinguishes this species from other larvae found in dog faeces, such as Crenosoma vulpis (Figure 2) (McGarry and Morgan, 2009).
The Baermann's test can be conducted easily in the practice laboratory, and although it should be left overnight for the best results, the majority of infected samples can be identified after an hour or so. The main problem with the Baermann's test is its low sensitivity, such that three consecutive faecal samples are usually recommended for diagnosis (Koch and Willesen, 2009). Other methods such as faecal flotation (Schnyder et al, 2011a) and faecal smear (Humm and Adamantos, 2010) are possible to conduct in the practice laboratory, but also suffer from limited sensitivity. Faecal samples can be sent to an external laboratory, but since comparable methods are used in those laboratories, the limitations are similar. In reality, treatment is often administered without a definitive diagnosis, and response to treatment used as a diagnostic aid. This has obvious disadvantages in the management of individual clinical cases, and also results in a lack of solid information on the extent to which the parasite is an issue in a practice catchment, which would be useful to inform future approaches to clinical cases. Local information of this sort is important because some practices in the UK experience a high level of A. vasorum challenge, while in others it is currently rare or unknown. Recent changes in epidemiology are discussed below.
The purpose of this review is not to provide comprehensive information on the parasite and the disease, since several recent reviews are available (Koch and Willesen, 2009; Morgan and Shaw, 2010; Helm et al, 2010; Traversa et al, 2010). Rather, this article aims to highlight recent changes relevant to practice in the UK. These centre on the changing range of the parasite, new diagnostic tests, and expanding treatment options.
Parasite spread: the worms go marching on
Historically, A. vasorum distribution has been characterised by a number of highly endemic areas, and low or zero prevalence outside those areas in foxes (Morgan et al, 2008), and few or no cases in dogs (Morgan et al, 2005). In the UK, such hot-spots have been established in Cornwall and in the Swansea area of south Wales for several decades, and in London and the south-east of England since the 1990s (Chapman et al, 2004). More recently, expansion appears to have occurred outside of those core areas. Elsewhere in Europe, similar expansion outside known endemic areas has been reported (Traversa et al, 2010), with new records in a series of countries. As in the UK, this is likely to be fuelled in part by increased awareness of the parasite, such that it is more likely to be recognised and reported, and also by specific studies aiming to demonstrate wide distribution. This process has undergone a step change in the past 3 years with the advent of new serological tests for detection of A. vasorum specific antigens and anti-parasite antibodies in dog serum (Schnyder et al, 2011b). In the UK, these tests have confirmed low prevalence in healthy dogs, and also a wide distribution in the southern part of the UK (Schnyder et al, 2013). This survey did not extend to the northern UK.
While apparent range expansion is certainly driven in part by increased surveillance and awareness, evidence does point to a real underlying spread. In the UK, veterinary practitioners and parasitologists have been aware of the parasite for a long time, and it can cause severe disease. It therefore seems unlikely that angiostrongylosis has always been common across the UK and was simply overlooked until recently. Cases in non-travelled dogs in northern England (Yamakawa et al, 2009) and Scotland (Helm et al, 2009) have been reported relatively recently, and the presence of the parasite in foxes (Philbey and Delgado, 2013) shows that transmission is now established in parts of the north. Very recent data show that prevalence in foxes has increased significantly in all endemic areas in the past 8 years, and that the previously negative areas of northern England and Scotland are now colonised (Morgan, unpublished data, 2014). In other countries in Europe and North America, a similar expansion appears to be occurring (Koch and Willesen, 2009; Conboy, 2011; Schnyder et al, 2013; Kistler et al, 2014), suggesting that underlying processes are in play leading to improved conditions for transmission of A. vasorum, maintenance of parasite populations in the wildlife host, and/or spill-over into the domestic dog population. These could include urbanisation of the red fox, increased dog movement, climate change, or a combination of these and other factors. Temperate climates appear to be associated with high A. vasorum prevalence in foxes (Morgan et al, 2009), and further expansion in the UK is to be expected.
Increased awareness and treatment of infection in dogs could suppress clinical disease even in areas in which the parasite is well established. A recent postal survey of practices in the UK indicates wide distribution of clinical cases, with a focus of higher reported incidence in south-east England, and another in south Wales (Kirk et al, 2014). Interestingly, no such focus was identified in south-west England, even though this is a well-established endemic area (Martin et al, 1993). Although good diagnosis, prompt treatment and appropriate worming regimens in dogs are unlikely to eradicate infection locally, given maintenance in fox reservoir hosts, it might succeed in decreasing clinical incidence in the long term.
New serological tests: implications for practice and research
The limitations of the Baermann test, in terms of sensitivity and also client compliance in collecting faecal samples, have limited its use in practice, and spurred the search for new, more sensitive and convenient tests. The blood of infected dogs carries detectable parasite-specific DNA (Jefferies et al, 2011), antigens (Schnyder et al, 2011b) and anti-parasite antibodies (Schucan et al, 2012). Detection of circulating A. vasorum antigen has formed the basis of a new, point-of-care test, AngioDetect™ (Idexx Laboratories, USA) (Schnyder et al, 2014). Reported sensitivity was 84.6% in detecting patent A. vasorum infection (Schnyder et al, 2014), and specificity 100%. The test could be used in many different ways in practice, most obviously in the diagnosis of infection in clinically affected dogs with suspicion of angiostrongylosis. In areas not known to have a high incidence of infection, it would be logical to use the test to screen cases with highly compatible clinical signs, such as coughing or dyspnoea in young dogs, or bleeding disorders. In practices with known high prevalence, these cases are likely to be recognised by clinicians with experience of the disease, and the test could be useful to confirm such cases. Testing could perhaps be even more useful in those areas to screen unusual presentations such as neurological, locomotor or gastro-intestinal disease, in which A. vasorum might be implicated but is not the primary differential. Other possible indications to test for circulating antigen include pre-anaesthetic screening, especially for young dogs, such as for neutering in endemic areas. A positive test would prompt treatment and a delay before undergoing surgery, to allow coagulation function to normalise.
Finally, routine use of the test in any of these ways should be accompanied by good recording and evaluation of results at practice level. This would enable a picture of the local level of threat to emerge. The monitoring process should be continuous as the level of threat is likely to change in many practices over the coming years. By entering AngioDetect™ test results online (www.angiodetect.co.uk), real-time mapping of positive results is also possible, with shared benefits for assessing regional risk.
New proven drugs
Fenbendazole has long been used to kill A. vasorum but must be administered daily for 7–21 days, and is not currently licensed for this purpose in the UK. Moxidectin and milbemycin oxime have also been shown to be effective (Conboy, 2004; Willesen et al, 2007) and are licensed for treatment of angiostrongylosis. Moxidectin 2.5 mg/kg spot-on (Schnyder et al, 2009) and milbemycin oxime 0.75–1.0 mg.kg orally (Böhm et al, 2014) have been shown to prevent patent angiostrongylosis by killing immature worms following experimental infection. This underpins licensed monthly use of moxidectin or milbemycin to prevent A. vasorum infection. Since label claims of different drugs are liable to change as evidence of efficacy in different circumstances is updated, it is advisable to stay aware of the current status of label claims, and not to rely for knowledge on journal articles, which can quickly become out of date on this subject.
Veterinary nurses in the vanguard
The spread of A. vasorum in the UK and other parts of Europe in recent years has fuelled increased awareness among veterinary surgeons, and the parasite is likely to be considered in the work up of a wide range of clinical presentations. As well as supporting case management, there are several areas in which veterinary nurses have a particularly valuable contribution to make at practice level. The first is in the collation of evidence from diagnostic tests, including laboratory and in-house Baermann's tests and the new AngioDetect™ test. This should be part of ongoing practice efforts to monitor the local epidemiological situation. Data so collected, in turn, should influence index of suspicion for angiostrongylosis in a wide range of clinical presentations. Furthermore, knowledge of the local epidemiological situation should influence advice given to clients on appropriate worming regimens. Even in high risk areas and individual dog profiles (e.g. young dogs in areas known to have high disease incidence), where recommendation is likely to be regular (monthly) use of a licensed preventive wormer, taking the time to discuss this with the owner is important in ensuring compliance and perceived value. This is the second area in which veterinary nurse input is key, since such conversations take time, which is often at a premium.
Developing individually tailored worming regimens for dogs is even more time consuming and requires detailed knowledge of risk factors and how they apply to each dog's profile. So far, only age has emerged as a strong and consistent risk factor for angiostrongylosis, with young dogs (< 18 months) much more likely to test positive (Koch and Willesen, 2009; Morgan et al, 2010). Results on breed predispositions are more variable, with Staffordshire Bull terriers and Cavalier King Charles spaniels identified to be at higher risk in some studies (Chapman et al, 2004) but not in others (Morgan et al, 2010). The behaviour of individual dogs is likely to influence risk of ingesting infected slugs, with activities such as scavenging, grass chewing, outdoor feeding, and off-lead exercise in areas inhabited by foxes, especially at night and in mild damp weather, all putatively increasing risk. However, data on the influence of behavioural factors on infection are few and circumstantial, and the ability to apply them to either decrease risk or guide appropriate anthelmintic intervention is questionable. What is clear is the lack of effective acquired immunity to re-infection, and the shared risk factors at household level. Any dog diagnosed positive should therefore be considered at high risk of re-infection, along with any house or kennel mates.
Conclusions
A. vasorum has spread in the UK in recent years, and given abundant fox populations and widespread dog movement, and climate change, it will probably continue to spread. However, better tools than ever are now available for diagnosing, treating and preventing infection. These should help not only to manage individual cases, but also to recommend effective and appropriate preventive worming regimens for dogs. Nurses have an important role to play in building information of local risk and communicating this to practice clients in support of evidence-based control.

