With all sick companion animals, the nutritional goal is for the patient to eat the designated diet in its own environment and in sufficient quantities. Critically sick animals can be in a hospital setting, where the added stress of being in a different environment can affect food consumption and metabolism. Many animals may require, or benefit from, a veterinary therapeutic diet but the initial goal is to ensure that the patient is receiving its daily calorific requirement from a nutritionally balanced diet.
Analgesia should not be forgotten as pain can reduce food intake in some animals. Hydration and electrolyte status must be maintained and corrected before any nutritional support can be initiated. The aim of critical care nutrition is dependent on the disease process and/or the individual's specific requirements. Each case must be considered according to its own specific requirements once a full clinical examination and history (including nutritional history) has been achieved (Freeman et al, 2011).
The sole aim can be defined as to prevent and/or treat malnutrition when present. To define nutritional aims in more depth, it is beneficial to split the aims into short-term and long-term goals. The short-term aims are to:
- Provide for any ongoing nutritional requirements (both in terms of energy and nutrients)
- Prevent or correct any nutritional deficiencies or imbalances
- Minimise metabolic derangements
- Prevent further catabolism of lean body mass. Long-term nutritional aims should include:
- Restoration of optimal body condition
- Provision of required nutrients to the animal within its own environment.
As disease processes change, and the animal's physiological and metabolic responses alter the nature of the nutritional support, both the short-term and long-term nutritional aims may be altered. When assessing animals for the preferred method of critical care feeding, the nutritional status of the animal needs to be evaluated. This should include body condition score (BCS), muscle condition score (MCS), hydration status, weight, hair coat quality, signs of inadequate wound healing, hypoalbuminaemia, lymphopenia and coagulopathy (Chan, 2005). Thought should be given to ‘fluid shifts’ in these animals as they can severely affect haematological values and the animal's weight. Factors that should be identified include electrolyte imbalances, hyperglycaemia, hypertriglyceridaemia or hyperammonaemia, as they will have large consequences on the nutritional critical care plan (Remillard et al, 2000). Adequate adjustments will be required to the feeding plan.
Energy requirements during sickness are based on resting energy requirement (RER) values (Box 1). This is because of the assumption that the patient is inactive and is often confined to a small area. Illness factors are no longer used, but nutritional assessments should be instigated to ensure adequate nutrition. Daily weighing of the patient, and accessing healing rates and lean body mass are good ways of identifying whether the patient is receiving sufficient calories (Freeman et al, 2011). As with all hospitalised patients, human and animal, malnutrition has been associated with increased infectious morbidity, prolonged hospitalisation and an increase in mortality.
Box 1.Calculations for resting energy requirements (RER)Calculations of enteral feeding amounts.
- Calculate the resting energy requirement (RER) of the animal RER = 70 x (bwt kg)0.75 for animals <2 kg or >45 kg, or 30 x (bwt kg) + 70
- Choose the specific diet, which is most beneficial for the patient and the method of feeding.
- Divide the energy content of the diet (kcal/ml or gram) by the energy requirement of the animal (kcal/day) to achieve the daily amount of food required.
- Divide the total amount to be given in a day by the total amount of feeding wished to be given, or by maximum volume of each feed.
The volume of liquid diets administered at each bolus feeding in dogs and cats should not exceed 10–12 mls/kg (including tube flush volumes) (Gajanayake, 2015). This is only an estimate and each animal should be judged on an individual basis. Tolerance to liquid diets is best when small feeds are delivered frequently.
Starvation and anorexia
Starvation can leave the animal severely emaciated, but the gastrointestinal tract will also become atrophied due to the inadequate nutrient supply. Intestinal villi become atrophied and the epithelial layers become thin and fragile (Mansfield et al, 2011). Bacterial translocation has the potential to occur in these cases. The gastrointestinal capacity to digest and absorb nutrients will become severely limited. The loss of lean body mass occurs from skeletal muscle and internal tissues.
Nutritional support of these animals is vital and required immediately. Initially hydration, electrolytes and acid–base status of the patient needs to be rectified. The diet chosen needs to be of a high digestibility, and primarily consisting of proteins and fats as the patient will use these nutrients over carbohydrates. The patient has the potential to be suffering from protein energy malnutrition (PEM) and the quality of the protein fed is important. On initiating nutritional support to the patient, small frequent meals are required, slowly building up over a period of time to the full daily nutrient and energy requirements, in order to prevent refeeding syndrome. PEM can occur during times of illness and when increased demands of protein and energy are required.
Clinical nutrition
Water (hydration levels)
The initial stage of nutritional support is correcting any dehydration, electrolyte replacement and normalisation of the acid-base status before starting assisted feeding. Initiating assisted feeding before the patient is haemodynamically stable can further compromise the patient. If oedema occurs or dehydration persists, recalculation of flow rates is required. Monitoring of hydration level indicators is required during intravenous fluid therapy. Daily maintenance fluid requirements are approximately 50–60 mls/kg of bodyweight (bwt)/day, or 2 ml/kg bwt/hour. If persistent vomiting or diarrhoea is present, these additional losses need to be factored in and urine output should be monitored.
Where dogs and cats are able to consume fluids without vomiting, the use of an oral rehydration fluid should be advocated. Unless contraindicated, a bowl or bucket of water should be placed in the animal's kennel or stable. If required, additional fluids can always be administered intravenously, or via feeding tubes.
Protein
PEM can occur during periods of illness, injury or even after routine operations. During these periods, the body shuts down systems to conserve its limited resources. Physiological changes such as a drop in blood pressure, reduction in cardiac output and a drop in oxygen consumption occur. This is clinical shock and is known as the ebb phase. Clinical shock is treatable but the length of time during which shock is suffered is variable and can ultimately be lethal. Following the ebb phase is the flow or hypermetabolic phase. During this second phase, the patient's body defence and repair mechanisms are initiated. This process is where anorexia and starvation differ (Michel, 2006).
Nutritional assessment and support with adequate protein levels for the life stage is vital; patients in a catabolic state will use the skeletal muscle proteins. Sufficient calories need to be supplied to the patient from fats and carbohydrates. This will ensure that proteins are not used as a source of energy. The quality of the proteins provided is of importance as is the digestibility.
Specific amino acids are supplemented to critical care diets. Glutamine is an important substrate for the increased levels of gluconeogenesis, used in rapidly dividing cells. Any deficiency in this amino acid has been shown to lead to gut mucosal atrophy, and an increase in bacterial translocation, due to a compromise of the mucosal barrier. This can lead to the suggestion that glutamine may behave as a ‘conditionally essential’ amino acid during severe illness. The essential amino acid, arginine, has a positive effect on the immune system and can subsequently improve survival times of septic patients. Many enteral diets are supplemented with both glutamine and arginine. A high dose of glutamine has a trophic effect on the gut mucosa.
Use of a novel protein source in these cases has been advocated. Philpott et al (1998) postulated that an intestinal inflammatory environment is capable of playing a role in the formation of food allergies. This has also been hypothesised by Cave (2006) and Fogle and Bissett (2007) in cases of inflammatory bowel disease (IBD).
Vitamins and minerals
The supplementation of vitamins and minerals for hospitalised patients will depend on the disease and its severity. Sodium, chloride, potassium, phosphate, calcium and magnesium should be used for short-term nutritional support. All animals that receive intravenous fluid therapy, with or without parenteral support, should have daily electrolyte levels monitored. If any polydipsia or polyuria is present, supplementation of the water-soluble vitamins is required (Elliott, 2015). Zinc aids in promoting wound healing and plays a role in protein and nucleic acid metabolism. Supplementation of nutritional support diets with zinc has been recommended.
Carbohydrates
Carbohydrates within any critical care diet needs to be of a very high digestibility. The quantities of fibre need to be kept to a minimum as this will decrease digestibility and bind up important nutrients. The crude fibre concentrations are generally very low at 3–6.9 g/1000 kcal and 1.2 g/1000 kcal in ‘recovery diets’ and liquid diets respectively (Becvarova, 2015). Carbohydrate levels in the diet need to be adequate enough to supply the required calories for recovery.
Fats
The quantity of fat in a critical care diet needs to be increased. A higher level of calories can be obtained from fat rather than from carbohydrates. The inclusion of omega–3 fatty acids can help decrease any inflammatory response. Liquid and recovery diets will contain on average 45–47% and 55–68% calories from fat, respectively (Becvarova, 2015).
Supportive feeding methods
Supportive feeding methods should be considered when specific areas of the alimentary canal need to be bypassed or if there is a history of any of the following:
- Greater than 10% weight loss
- Decreased food intake
- Anorexia
- Increased nutrient demands due to trauma or surgery
- Increased nutrient losses resulting from vomiting, diarrhoea, burns or scolds
- Acute exacerbation of chronic disease.
The ideal diet in these cases should be highly palatable, digestible and have a high energy density. A relatively high percentage of energy should be produced as a result of protein and fat intake rather than carbohydrates. The percentage of total calories from digestible carbohydrates in the liquid enteral diets is typically restricted to 21–25% (Becvarova, 2015).
Critical care diets for cats and dogs are available in three different forms: powdered, liquid and moist diets. Moist diets can have thixotrophic consistencies; when mixed, they become thinner as their viscosity decreases.
The use of assisted feeding methods does have great advantages but care of the feeding tube is vital. The artificial opening through the abdomen into the gastrointestinal tract through which the tube is inserted is referred to as a stoma. The stoma must be treated as a surgical wound and cleaned daily with normal saline or cooled boiled water for the first 7–14 days, or until it is healed.
Dressings around tubes are not necessary unless indicated, for example if the stoma site is infected or the animal is interfering with the insertion site (Figure 1). The tube should always be checked to ensure that it is still situated within the gastrointestinal tract prior to administering any diet. This can be achieved by slow administration of water into the tube, assessing for any coughing or sneezing.
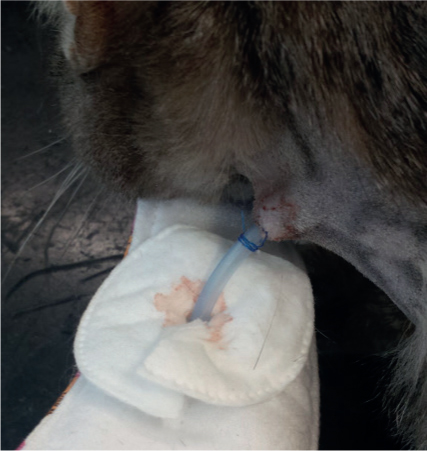
Radiography can also aid in assessing location of the tube but cannot be achieved at every feeding. A capnograph can be used, and if a trace is seen that is similar to that of an anaesthetised patient, it implies the tube has moved into the trachea. There is potential for this to happen, especially in cases where naso-oesophageal feeding tubes are used.
Encouraging animals to eat
The process of encouraging animals to eat should never be forgotten. Voluntary intake can be established in a number of cases by taking time out to personally encourage the animal to eat. Grooming can actively encourage the animals (especially dogs and cats) to eat, removing any nasal discharge that is blocking their sense of smell. Tender loving care, providing competition, hand feeding, a selection of different diets, a different environment and, in some cases, offering the animal some of the food that you are eating if it is suitable can also help. This last method works especially well in dogs.
Microenteral nutrition
Microenteral nutrition is the delivery of very small amounts of water, electrolytes and easily absorbable nutrients directly into the gastrointestinal tract. This method is often under-used in veterinary practices but allows for the nutritional requirements of the intestinal mucosa to be met. This helps to preserve the intestinal blood flow, as well as the mucosal barrier and its immune function (Buffington et al, 2004). Initial volumes of 0.05–0.2 ml/kg bwt/hour are recommended and will add exceptionally little to the volume of fluids normally produced by the stomach. Gradual increases can occur to 1–2 ml/kg bwt/hour, over 24–48 hours. Enteral solutions that can be used include oral rehydrating solutions, and those containing glutamine. This method of nutrient delivery can be administered orally or via tubes.
Naso-oesophageal tubes
Naso-oesophageal tubes (Figure 2) are generally well tolerated by cats and dogs and are suitable for short-term nutritional support, usually 3–7 days, though longer periods have been documented (Chan, 2015). Contraindications for the use of these tubes include unconsciousness, vomiting, disease or dysfunction of the pharynx, larynx and nares, swallowing reflex, oesophagus and stomach. The preferred placement of the naso-oesophageal tube is in the caudal oesophagus, rather than the stomach, as it reduces the risk of reflux oesophagitis (Gajanayake, 2015).
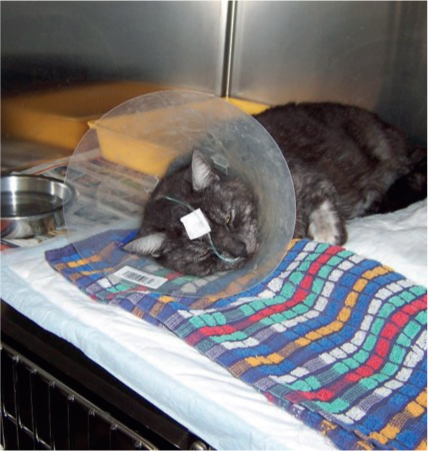
Once the tube has been placed, a small amount of water can be injected slowly into the tube to see if a cough reflex is induced, indicating aspiration. Lateral radiographs can also identify that the tube has been correctly placed. Due to the tube's narrow bore, blockages can occur. Administration of 5–10 mls of water into the tube following the liquid diet should help to prevent any blockages. If blockages do occur, small amounts of carbonated drinks, cranberry juice or solutions of pancreatic enzymes have shown to aid in their removal. Pre-feeding administration of water is required to ensure that the tube is still positioned correctly.
Oesophagostomy tubes
Oesophagostomy tubes (Figure 3 and 4) are commonly used in cats, which have suffered facial trauma, usually after a road traffic accident. These tubes are of use when bypassing of the nose and mouth is required to administer nutritional support. Animals that do not tolerate naso-oesophageal tubes well can have oesophagostomy tubes placed. Aseptic placement of the tube under general anaesthetic is required. Frequent cleaning and inspection of the tube is necessary under aseptic conditions. Complications can include airway obstruction, infection and damage to the cervical nerves and blood vessels.


Percutaneous endoscopic gastrostomy tubes
Placement of percutaneous endoscopic gastrostomy (PEG) tubes is required under general anaesthetic and should remain for at least 5 days prior to removal. PEG tubes are used when long-term nutritional support is required, and when oesophageal problems are present (for example megaoesophagus). Adhesions between the gastric serosa and the peritoneum can form within 48–72 hours. It should be noted that in malnourished patients, these adhesions might take longer to form. Once the tube is placed, only a third of the calculated daily energy requirements should be administered; on day two, two-thirds and by the third day, the full amount. Feeding through the PEG tube can commence 4 hours after its insertion. If patients are unable to take fluids orally, mouth care should be encouraged at least every 4 hours. This involves ensuring that the mucous membranes remain moist, and that bacterial infections are prevented. This can be managed using oral hygiene gels that contain chlorhexidine designed for dental care.
The procedure of removal of the gastrostomy tube in animals over 10 kgs is to cut the catheter off flush with the skin after pulling it taught. The catheter tip is then passed in the faeces. The resulting gastrocutaneous fistula will rapidly heal if kept clean.
Conclusion
Nutritional intervention is better when delivered early, it should be considered for all cases, and can have many positive outcomes to the overall health and quality of life for the pet.
Key Points
- A nutritional assessment should always be performed on every patient to ensure adequate nutrition is being achieved.
- Resting energy requirement (RER) calculations only calculate the number of calories to be fed but do not take nutrients into consideration.
- Assisted tube feeding should always be considered as an option.


