Guinea pigs and rabbits are obligate nasal breathers that are unable to regurgitate, and therefore have a minimal risk of aspiration under general anaesthesia (Cantwell, 2001). This unique physiological trait has been a longstanding reason for the use of an appropriately fitting face mask for the provision of oxygen and inhalant anaesthetic in place of an endotracheal (ET) tube. However, using a face mask in these species on the basis that they cannot regurgitate negates the many other benefits of intubation. Tracheal intubation is indicated for maintaining and controlling a patent airway in an unconscious patient and to provide mechanical ventilation in a hypoventilating or apnoeic patient (Cantwell, 2001). Intubation also allows for the use of airway monitors and for an unobstructed surgical approach to the cranial aspect of a patient (Johnson, 2010). However, intubation is easier said than done. Rodents and rabbits have a small oropharyngeal cavity, large tongue, large molars, a large soft palate that obscures the epiglottis, a small larynx, a predisposition to laryngospasm and profuse respiratory secretions that can contribute to the risk of aspiration (Johnson, 2010). Guinea pigs also have a palatial ostium; the soft palate and tongue are continuous, forming a ring of tissue between the pharynx and oropharynx. This tissue is highly vascular and can become easily traumatised, resulting in profuse haemorrhaging (Cantwell, 2001). Difficult, prolonged, or traumatic intubation can result in laryngeal or tracheal inflammation, which can lead to obstruction and death. To minimise the risk of this iatrogenic damage, intubation attempts should be limited to two or three tries (Pollock, 2011).
Pre-induction considerations
Small exotic mammals are prone to stress in hospital environments and should be given time to become accustomed to their unfamiliar surroundings. These species have high sympathetic tone that can often override sedation and predispose them to vasoconstriction and arrhythmias (Cantwell, 2001). A warm, dark, and quiet environment prior to anaesthetic induction is beneficial. Because of their high metabolic demands and likelihood of developing hypoglycaemia or ileus, prolonged preoperative fasting of rodents and rabbits is not recommended (Cantwell, 2001). Guinea pigs and rabbits practice coprophagy and have a large caecum, so fasting will not significantly reduce their abdominal contents (Cantwell, 2001). While neither guinea pigs or rabbits experience vomiting, guinea pigs may retain food in their pharynx which greatens the risk a vomit-like event and aspiration (Cantwell, 2001). Withholding food and water and flushing the oral cavity of remaining food at time of sedation, approximately 10–20 minutes prior to induction time, should become a routine preanaesthetic consideration.
Rabbits can struggle and kick when restrained, increasing the risk of fractures and dislocations if mishandled or dropped. Rabbits may also have underlying or subclinical respiratory disease (e.g. Pasteurella spp.). Their large abdomen that is often full of ingesta can decrease tidal volume and cause further respiratory compromise (Cantwell, 2001). Careful restraint, preoxygenation and the use of medications that cause the least respiratory depression is ideal prior to airway assessment and intubation. Intravenous (IV) access is best gained through the marginal ear vein using a 24 or 26 gauge catheter (Briscoe and Syring, 2004).
Similarly, guinea pigs may also have underlying or subclinical respiratory disease and have a large abdomen and full gastrointestinal tract (Cantwell, 2001). Respiratory secretions can be thick and profuse, and manual wiping with a gauze swab may be indicated regardless of intubation or masked anaesthetic maintenance (Cantwell, 2001). Anticholinergics, while historically used to reduce these secretions, tend to thicken the secretions and increase the risk of mucous plug formation instead (Johnson, 2010). Intravenous access is limited, with the use of a 26 gauge catheter in the cephalic vein (Briscoe and Syring, 2004). Intramuscular or subcutaneous injections are preferred for short or routine procedures.
Rats and other small rodents can have variable anaesthetic responses based on weight, age and strain. Subclinical respiratory disease is common, and intubation is impractical in any rodent smaller than a rat (Cantwell, 2001). The placement of monitoring equipment can also be challenging because of their smaller size, with most monitoring being observational (Cantwell, 2001). Intravenous access is possible in rats via the lateral tail vein, but becomes impractical in smaller rodents because of their vein size (Briscoe and Syring, 2004).
Endotracheal tube selection
Commercial ET tubes tend to be an appropriate length for dogs and cats, and therefore too long for small exotic mammals. Excess length will increase mechanical dead space, contributing to carbon dioxide (CO2) accumulation and respiratory acidosis (Briscoe and Syring, 2004). Unilateral intubation of one side of the respiratory tree can also occur (Figure 1), contributing to atelectasis of the non-intubated side (Johnson, 2010). Standard ET tubes should be manually shortened to ensure the length reaches from nares to the point of the scapula, which is approximately cranial to the point of bronchial bifurcation (Briscoe and Syring, 2004). As a result of the already small diameter of the rodent or rabbit trachea and the potential for tracheal trauma, uncuffed ET tubes of appropriate sizes are preferred (Cantwell, 2001). Rabbits will usually require a 2–3.5 mm ET tube. Guinea pigs will require a 2–2.5 mm ET tube. Rats can be intubated using a 14 or 16 gauge IV catheter with the stylet removed (Cantwell, 2001).
Intubation techniques
Various intubation techniques for small exotic mammals have been discussed in literature; some requiring specific equipment and all requiring thorough practice (Cantwell, 2001; Briscoe and Syring, 2004; Lennox and Capello, 2008; Johnson, 2010; Miranda et al, 2017). These techniques can be divided into blind intubation or visual intubation, and visual intubation can be further subdivided into direct or indirect visualisation.
Blind intubation
Blind intubation involves positioning the head and neck so that the pathway from oropharynx to the trachea is straight. In theory, an ET tube can be placed without visualisation of the larynx. This technique may require laryngeal palpation, close observation of the patient's response (e.g. coughing) or listening for and observing the presence of air movement through the ET tube post-intubation (Pollock, 2011). The use of capnography to observe end tidal CO2 will also confirm correct placement (Johnson, 2010). The risk of laryngeal and tracheal trauma is greatly increased with this technique, as is the risk of aspiration if the presence of food or respiratory secretions within the pharynx has not been identified and resolved (Johnson, 2010).
Direct visual intubation
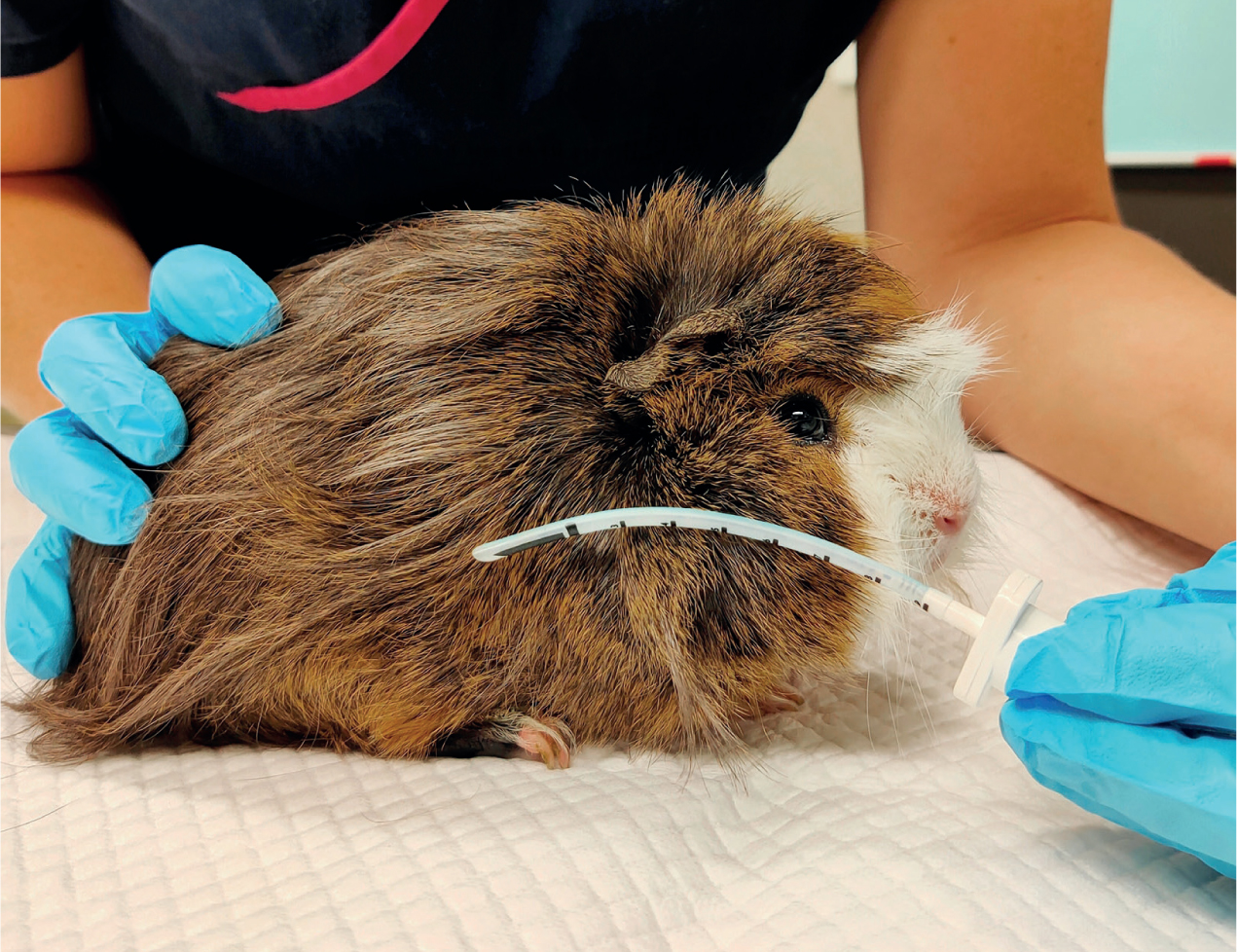
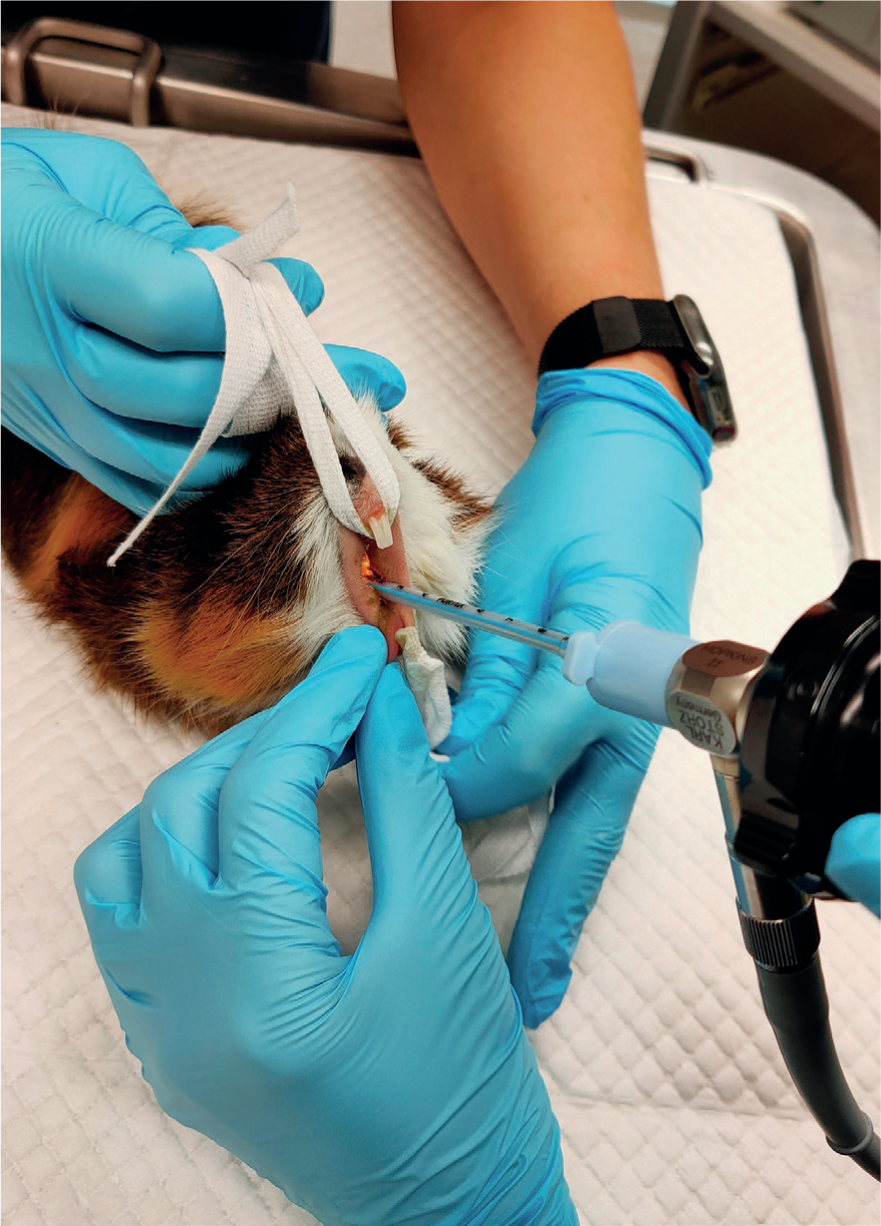
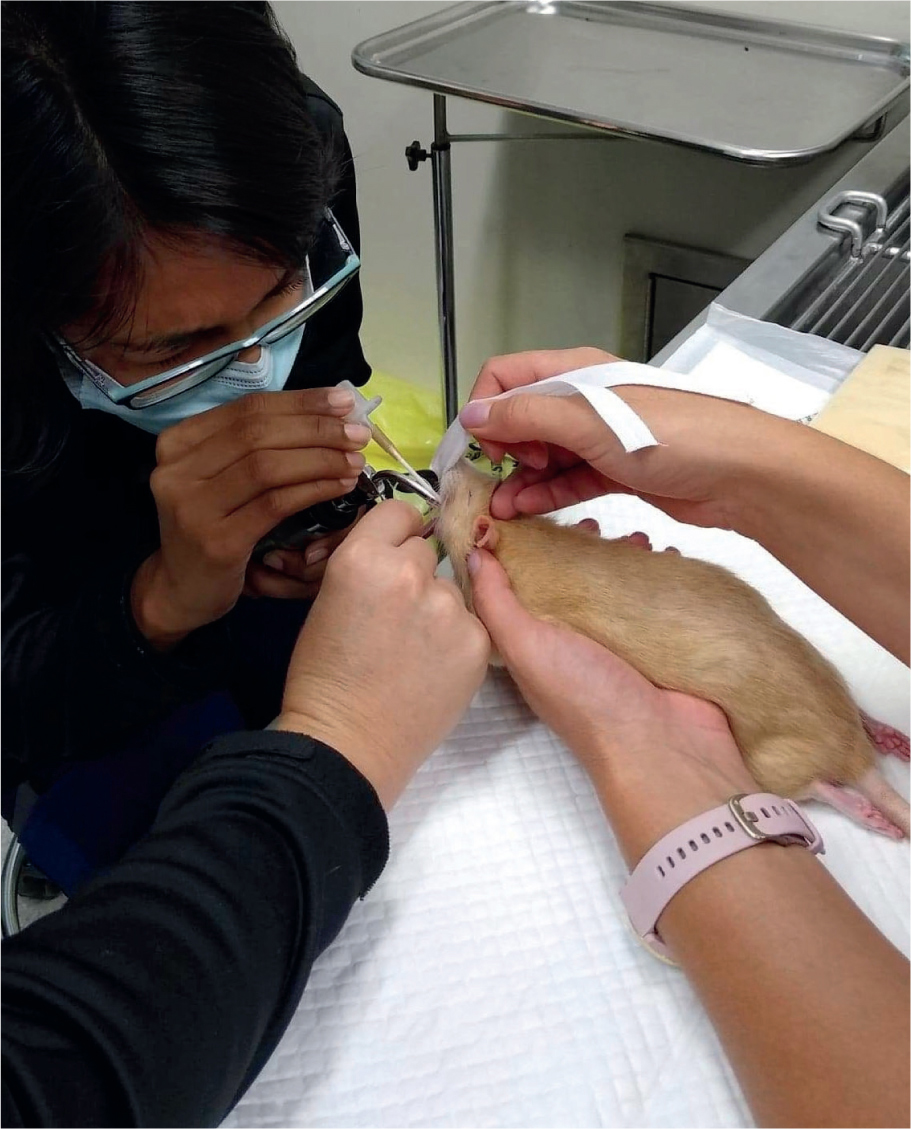
Visualisation of the glottis and larynx can be achieved through hyperextension of the head and neck. For adequate visualisation into the small oropharyngeal cavity, an assistant may be necessary for lifting up and pulling down the maxilla and mandible respectively using an ET tube tie placed behind the upper and lower incisors (Figures 2 and 3) (Pollock, 2011). An oral speculum may also need to be used (Figure 4). A small-bladed laryngoscope can be used to depress the tongue and elevate the soft palate. Once the vocal cords have been visualised, lignocaine can be applied to the laryngeal mucosa through an atraumatic catheter of appropriate length to decrease the risk of laryngospasm and either an ET tube or an atraumatic stylet (such as a polypropylene urinary catheter) acting as a guide wire can be placed into the trachea (Pollock, 2011). Being narrower than the ET tube, a stylet or catheter tip will easily advance into the trachea and the ET tube can then be threaded over the top and guided through the vocal cords. Similarly, a canine otoscope can be used in place of a small laryngoscope (Cantwell, 2001). Once adequate visualisation of the vocal cords has been achieved, a polypropylene urinary catheter can be guided down the otoscope and advanced into the trachea. The otoscope can then be removed, and an ET tube can be threaded over the top of the catheter. Once intubated, the catheter is then removed.
Indirect visual intubation — endoscopic intubation
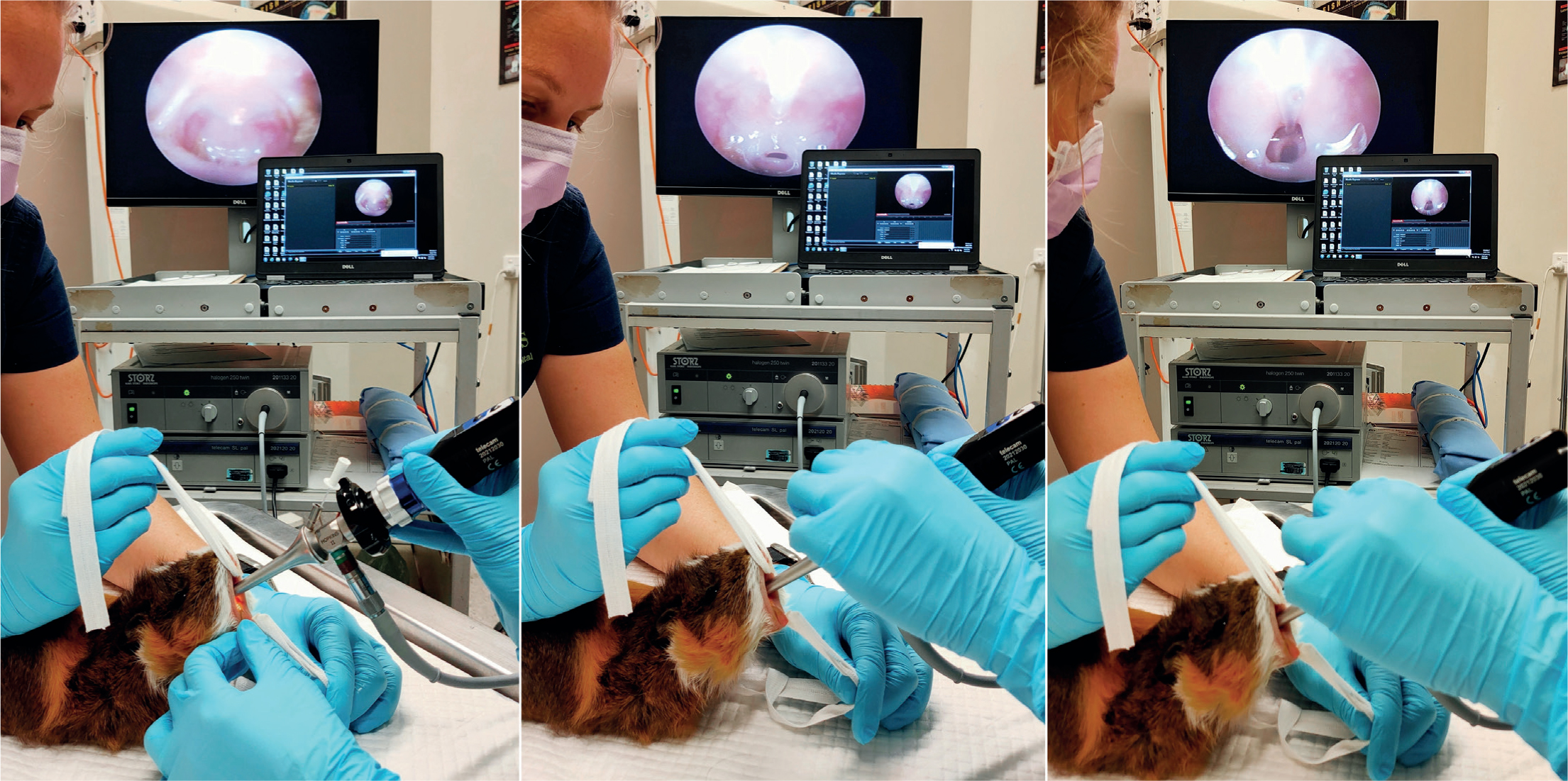
An endoscope can be used to indirectly visualise the glottis, provide a channel for the application of lignocaine on the laryngeal mucosa, or be used as a stylet to guide an ET tube into the larynx (Johnson, 2010). Three types of endoscopes are most frequently reported and used for small exotic mammal intubation: the 2.7 mm Hopkins rod lens telescope, the 1.9 mm Hopkins rod lens telescope, or the 5 mm rigid otoscope with a 5 Fr integrated working channel (Karl Storz Endoscopy Australia Pty. Ltd. Macquarie Park, NSW) (Johnson, 2010). The rigid otoscope is ideal for assessing the oral cavity for food or respiratory secretions, visualising the soft palate and glottis, for the application of lignocaine to the larynx via an atraumatic catheter through the 5 Fr channel, and for ‘side-by-side’ intubation. The 1.9 mm and 2.7 mm endoscopes are appropriate for ‘side-by-side’ intubation with any sized ET tube, or for ‘over-the-top’ guided intubation using ET tubes with an internal diameter of 2 mm or greater, and 3 mm or greater respectively (Figure 5). All three endoscopes are rigid, allowing for the soft palate to be pushed and for small exotic mammals on a lighter plane of anaesthesia to chew on the endoscope without damaging the equipment (Johnson, 2010). These endoscopes can incorporate a light source through a fibre optic light cable. Endoscopic intubation may also incorporate a camera and video monitor, thus becoming videoen-doscopic intubation (Miranda et al, 2016).

Rabbits can be successfully intubated using the rigid otoscope for visualisation and lignocaine application, followed by an ‘over-the-top’ intubation using the 2.7 mm endoscope and a 3–3.5 mm ET tube or ‘side-by-side’ intubation using the rigid otoscope for visualisation (Figure 6). The glottis of a rabbit is small, the tongue easily hides the larynx, and laryngospasm is a common occurrence (Lennox and Capello, 2008). The rabbit trachea is long and unilateral intubation is unlikely, but manual shortening of the ET tube is essential as trauma of the trachea at the point of bifurcation can occur (Briscoe and Syring, 2004). Insertion of an ET tube into the trachea is easiest during inspiration, when the vocal cords are open and not as likely to deflect the end of the ET tube into the oesophagus (Cantwell, 2001). During intubation, supplemental anaesthetic gas can be provided directly to the nares without obstructing the oropharyngeal cavity (Pollock, 2011). This is particularly important for maintaining an adequate depth of anaesthesia to prevent the patient from moving and traumatising themselves on the scope (Johnson, 2010).
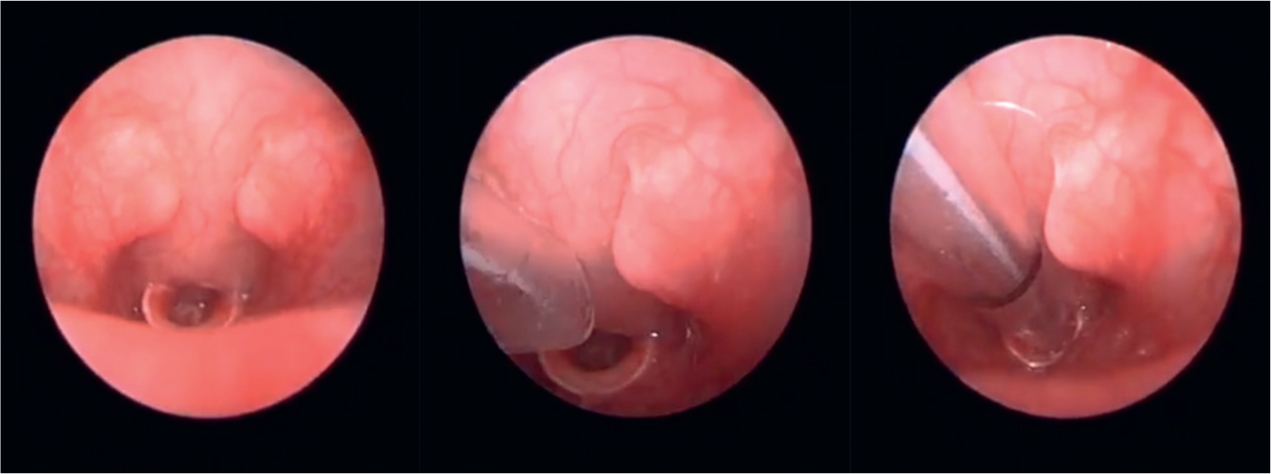
Guinea pigs can be successfully intubated in a similar manner to rabbits by using the 1.9 mm endoscope (Figure 2). The success of ‘side-by-side’ intubation is limited by their narrow palatial ostium composed of soft palate, palatoglossal arches, and tongue (Cantwell, 2001). Guinea pigs also tend to profusely hypersalivate under anaesthesia, which can interfere with visualisation. When using the rigid otoscope for visualisation, these secretions should be observed and wiped away with gauze swabs (Cantwell, 2001). The atraumatic catheter used for lignocaine application serves the secondary purpose of dislodging the soft palate from obscuring the epiglottis (Figure 3) (Johnson, 2010).
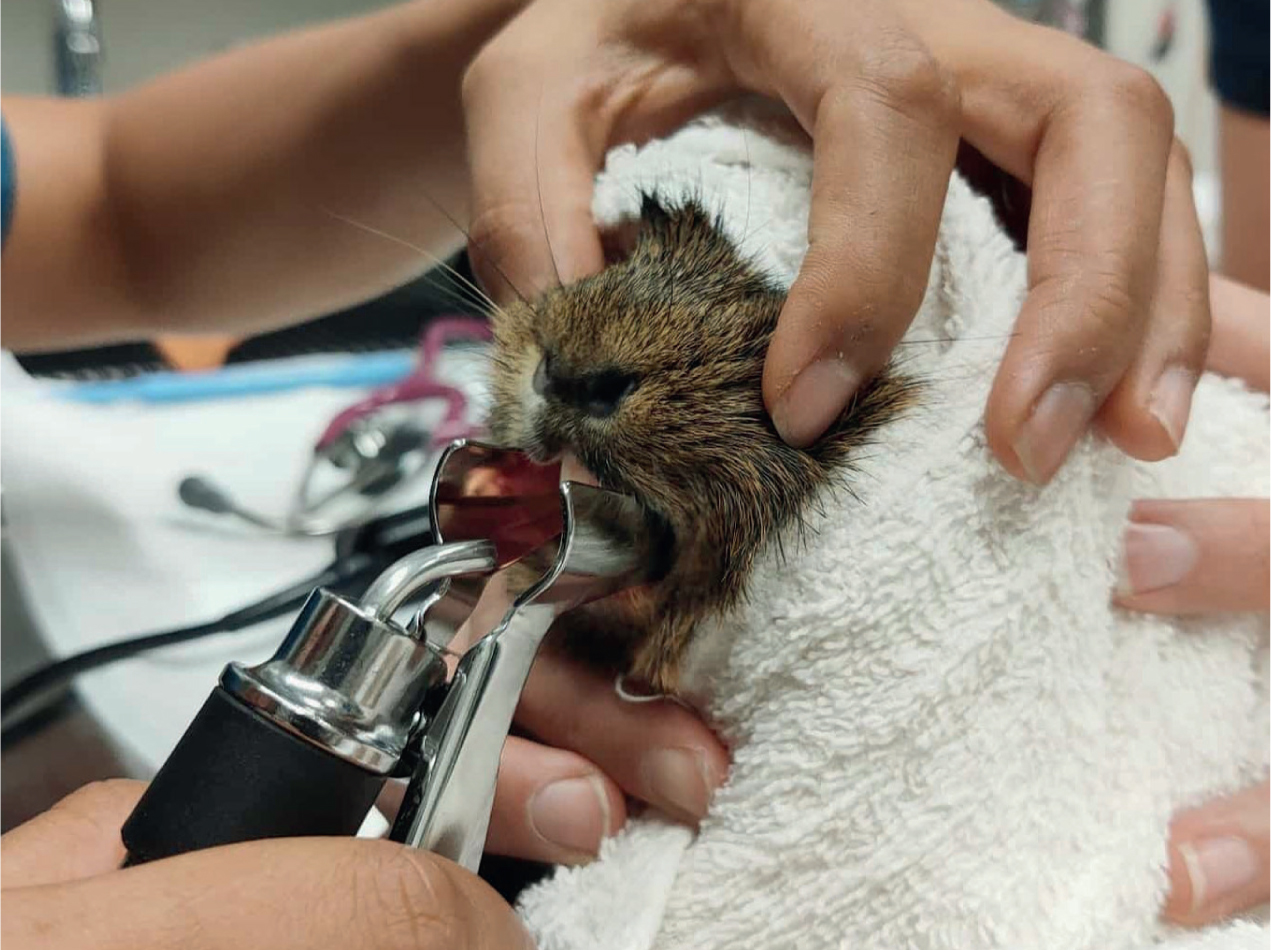
Rats can be intubated using the ‘side-by-side’ technique with either a 1 mm ET tube or makeshift ET tubes fashioned out of intravenous or urinary catheters (Cantwell, 2001). Endoscopic intubation of rats is limited because of their small oropharyngeal cavity, even smaller larynx, and vocal cords that move at high frequencies (Johnson, 2010). However, the illumination and magnification of the field of view from the scope lens to a video monitor can greatly aid in visualisation for intubation (Miranda et al, 2016). Rats can also be easily intubated through direct intubation (Figure 7). Their mouth opens widely with a small laryngoscope blade and their glottis can be easily visualised with good illumination (Briscoe and Syring, 2004). Intubation becomes difficult and impractical in mice and other exotic mammals smaller than a rat. It is possible to intubate mice with the use of a small light source, anything atraumatic and small enough to enter the oral cavity and depress the tongue without obscuring the glottis (Figure 8), and small gauge intravenous catheters converted into makeshift ET tubes; these smaller gauge catheters are prone to obstruction with respiratory secretions, resulting in a non-patent airway and the potential need to extubate and switch to masked anaesthetic maintenance perioperatively (Cantwell, 2001).

Conclusions
Endotracheal intubation can be challenging in small exotic mammals, but it is by no means impossible. The benefits of maintaining a patent airway far outweigh the training and equipment necessary to try. By normalising intubation as a part of the routine standard of care for smaller patients, the same high standard of intensive anaesthetic management afforded to dogs and cats can be replicated in rodents, guinea pigs and rabbits.
KEY POINTS
- Endotracheal (ET) intubation of small exotic mammals, while difficult, provides high quality intensive anaesthetic management.
- Stress, anatomical variation, variable reactions to drugs and underlying disease play a major role in the outcome of an anaesthetic and should be taken into consideration.
- Standard ET tubes are designed for dogs and cats, and require manual shortening and manipulation for use in small exotic mammals.
- Various intubation techniques exist, some requiring special equipment and all requiring thorough practice.
- Direct or indirect visual intubation techniques should become the routine standard of care for small exotic mammal anaesthesia.


