Feline lungworm has long been a high-profile problem in veterinary medicine. The offending parasite has overwhelmingly been considered to be Aelurostrongylus abstrusus, with many early publications citing this as the sole cause of feline lungworm.
However, is has become apparent that two other species can infect the bronchial tract of domestic cats: Eucoleus aerophilus (also known as Capillaria aerophila); and Troglostrongylus brevior. Recent reports have stimulated a scientific interest in knowing more about these parasites and more about lungworm disease in cats.
This interest is necessary to encourage and increase the understanding of the similarities and characteristics, ecological and epidemiological, of these nematodes so efficacious control programmes can be developed.
In this article, the authors review the disease and the parasites to give veterinary professionals a better understanding of it.
A. abstrusus, E. aerophilus and T. brevior
All three of these parasites are nematodes; A. abstrusus and T. brevior are members of the metastrongyloidae family and E. aerophilus is from the trichuridae family. The cat lungworm (A. abstrusus) historically has been regarded as the most important lung parasite in domestic cats in terms of species specificity, knowledge, geographical distribution and its role in feline medicine, with adult stages found in nodules in the bronchioles, alveolar ducts and alveoli of infected hosts. E. aerophilus has long been found in wild carnivores but, in recent years, it has also been reported in domestic cats and even humans. T. brevior has been regarded as a parasite of wild felids, but reports of infection in domestic cats have been increasing, mostly in young animals (Figure 1) (Diakou et al, 2014; Traversa and Di Cesare, 2016).
Life cycle and pathogenicity
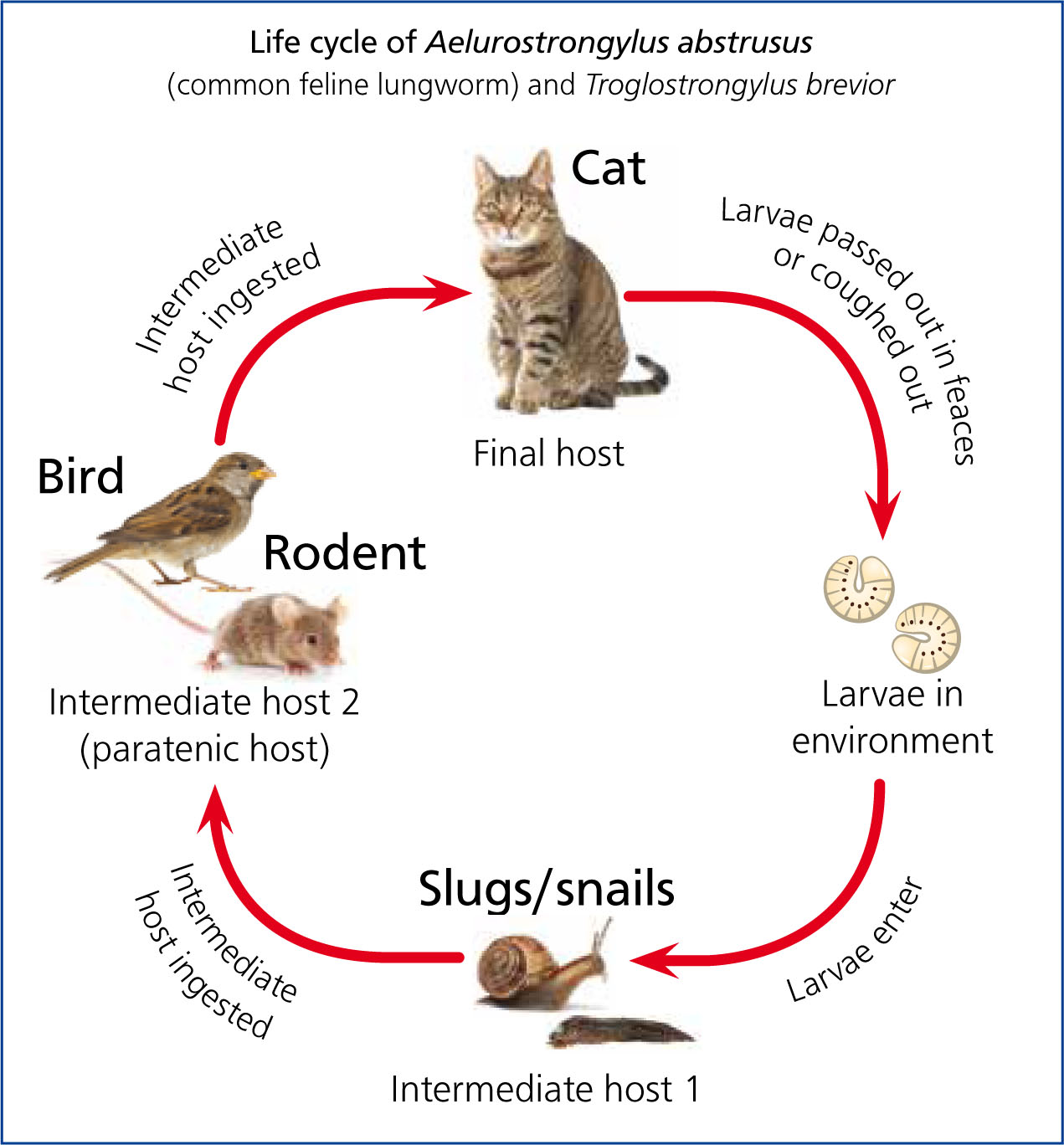
A. abstrusus has an indirect life cycle (Figure 2), with more than one host. Adult females lay eggs on the definitive host (cats). These hatch and the first stage larvae (L1) migrate and reach the pharynx to be coughed up then swallowed; they pass into the environment through the faeces. L1 then develop in snails to reach the L3 infective stage; this period of development depends on the environment temperature. Cats become infected by ingesting intermediate hosts or, more likely, facultative intermediate or paratenic hosts, such as birds and mice, which have ingested the snails (Traversa and Di Cesare, 2016).
Clinical signs are not always noticeable, as the disease may be subclinical or asymptomatic. In adult animals, where the mild forms with a low parasite burden can be seen, the condition may be self-limiting, with respiratory signs disappearing within weeks. Symptoms are due to the inflammatory response triggered by the eggs shed by the adult females and the migration of L1 along the bronchial tree, producing lesions in the alveoli, bronchioles and local arteries. However, in young, immunosuppressed or debilitated animals, signs such as mild to intense cough, sneezing, mucopurulent nasal discharge, dyspnoea, openmouthed abdominal breathing and even death can occur. (Traversa et al, 2010).
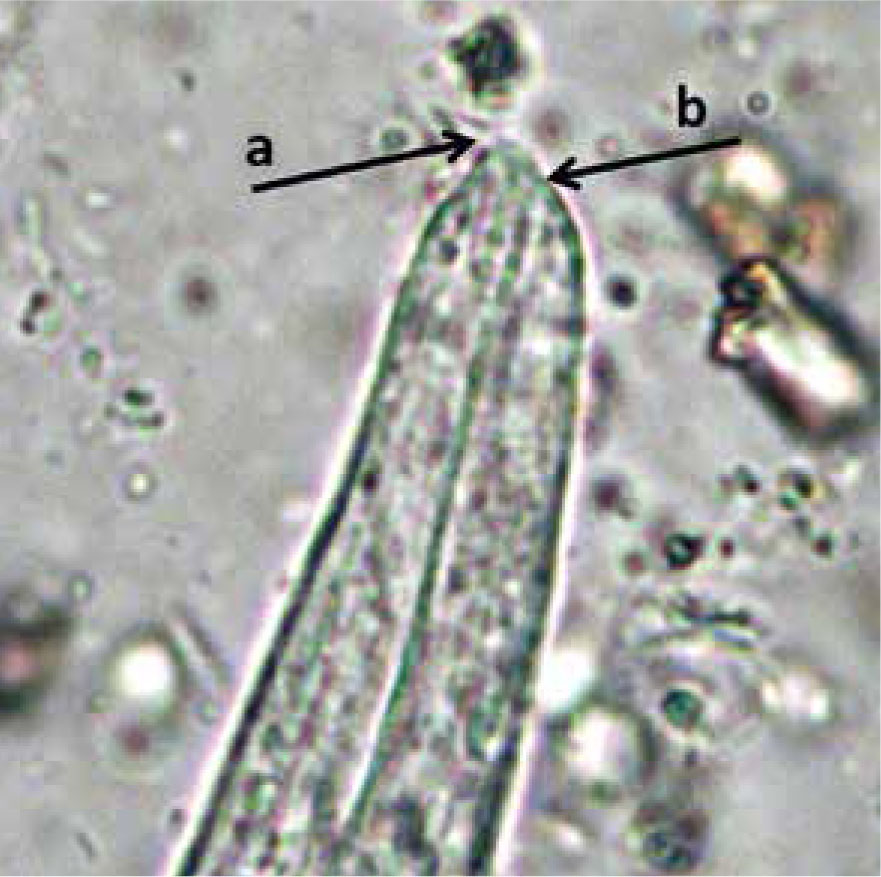
The life cycle of T. brevior is also indirect, with terrestrial molluscs being intermediate hosts, when L1 (Figure 3) develop to L3, and small mammals such as mice acting as paratenic hosts. The larvae of this nematode can develop in a variety of snails and slugs, becoming infective between 8 days (if the environmental temperature is 22–27°C) and 40 days (if the environmental temperature is 4–8°C).
A matter to consider when developing control programmes is that A. abstrusus and T. brevior share both an ecological niche and an intermediate snail host (Helix aspersa). Infected snails can infect naive snails and L3 in dead snails seek live snails to increase their chances of completing their life cycle. A. abstrusus L3 can survive for up to 36 days at 4°C and 14 days at 26°C; T. brevior L3 can survive for up to 28 days at 4°C and eight days at 26°C.
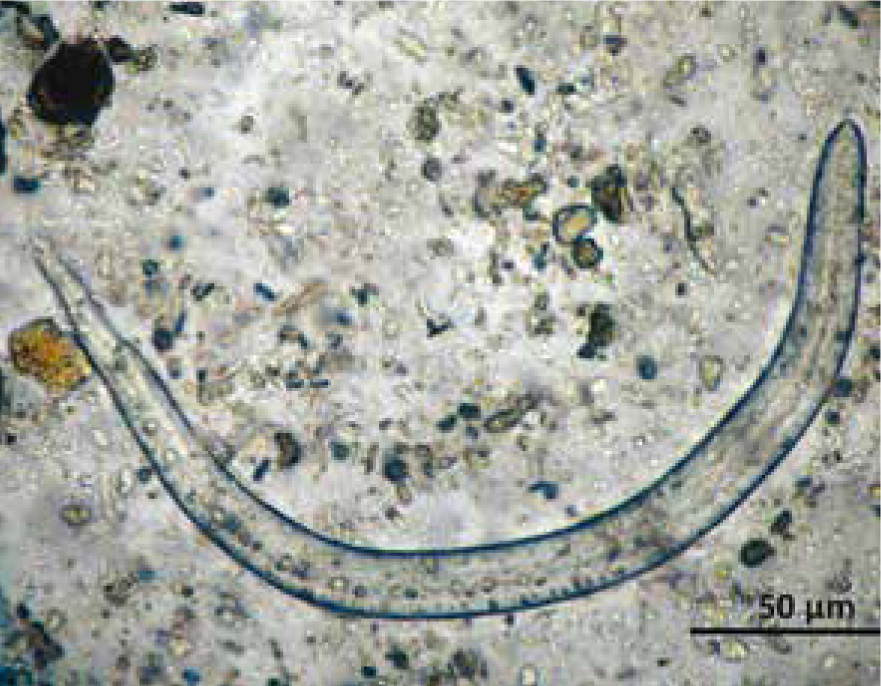
In contrast, E. aerophilus (Figure 4) has a direct cycle. After the adults mate, the female releases eggs, which are coughed up then ingested by the infected animal, then passed in the their faeces. After the embryos have developed, over a period of 1–2 months, the eggs reach infectivity. Animals can then become infected if they ingest eggs or, potentially, earthworms that have ingested eggs but the earthworm's role as a paratenic host has not been convincingly established (Traversa and Di Cesare, 2016).
The characteristic clinical sign of infestation is chronic bronchitis, which can range from bronchovesicular sounds to inflammation, sneezing, wheezing and chronic dry cough. However, if concomitant bacterial infections occur, a dry cough may become humid and productive and bronchopneumonia and respiratory failure occur (Traversa et al, 2010).
Troglostrongylus
The Troglostrongylus genus covers four metastrongylids that are found in wild felids — T. subcrenatus, T. brevior, T. troglostrongylus and T. wilsoni — each of which inhabit the trachea and bronchopulmonary tract of their definitive host (Brianti et al, 2012a).
Historical permutations of the genus contained only three of the above four species, with the majority of the species, most notably T. subcrenatus, being initially and erroneously classified within the genera of Haemostrongylus and Bronchostrongylus due to the morphology of the dorsal rays of the bursa of male specimens (Cameron, 1931; Dougherty, 1951). However it was reclassified by Fitzsimmons due largely to the spicule and cephalic morphological similarity between the two genera (Fitzsimmons, 1964).
Members of the Troglostrongylus genus have long been neglected as parasites of domestic cats, mainly because of their being considered largely a lungworm of wildcats, their perceived lack of pathogenicity in adult cats and a general lack of publications on this. The information on their prevalence among domestic cats in Europe is limited to a handful of publications over the last half a century. Consequently, the impact of this neglected genus of lungworm has yet to be fully elucidated.
Nonetheless, it can be inferred from the literature that T. brevior is an emerging feline pathogen that could be a future problem for domestic cats and veterinarians. Because of this, interest in this parasite among researchers has increased over the past 5 years or so, stimulating discussion on the extent to which this parasite could affect domestic cats.
Epidemiology of T. brevior: from a disease of wild felids to the emergence of domestic cat infection
Traditionally, A. abstrusus and E. aerophilus were considered the only metastrongylids to affect domestic cats. However, in recent years, there has been an emergence of cases and literature reporting the presence of T brevior in the domestic cat population (Brianti et al, 2012a; Diakou et al, 2015). The parasite had long been considered restricted to wildcats, with up to 63.6% of the wildcat population of southern Italy being infected (Brianti et al, 2012b; Otranto et al, 2013).
It is not yet clear whether wildcats are more susceptible to infection by T. brevior than domestic cats. However, it is probable that wildcats' dependence on hunting for food makes them more likely to ingest the worms' paratenic host which puts them at a greater risk of infection. It is also a well known observation among cat owners that domestic cats hunt but do not consume birds and rodents, which may also account for their lower incidence of infestation. However, it cannot be said that hunting practice accounts entirely for the bias towards wildcats, since this would apply to A. abstrusus, infestation, as they share an almost identical life cycle. This contrast in incidence is not apparent with A. abstrusus, with Aelurostrongylus infestation being epidemiologically significant in domestic cats (Diakou et al, 2015).
A correlation has been seen between the distribution of wildcats and the presence of T. brevior in local populations of domestic cats (Diakou et al, 2015). The earliest documented occurrences of T. brevior were in feral cats of Palestine and Italy in the mid 20th century (Gerichter, 1947; Paggi, 1959). Since 2010, the parasite has been documented in domestic cats, primarily in European islands (Crete, Sardinia, Ibiza and Sicily) and southern Italy (Brianti et al, 2012a; Diakou et al, 2014; 2015).
It may not, however, be accurate to say that the increase in cases of T. brevior is due to its recent emergence in domestic cats. It is possible that the shortage of historical cases is due to T. brevior being misidentified as A. abstrusus, due to the similarity of the first stage larvae used for diagnosis (Traversa and Di Cesare, 2016). As such, the epidemiology of this genus may have been misrepresented in past literature. This observation, however, is of some contention, and a number of opinion articles have been published on this matter (Otranto et al, 2013; Traversa and Di Cesare, 2013; Traversa, 2014).
As there is no conclusive evidence of misidentification of feline lungworms in the literature, a strong case could be made for the position that the increased number of reports of T. brevior in domestic cats demonstrates a rise in incidence. It is not an unknown for previously rare parasitic nematodes to spike within a host population, as observed with Angiostrongylus vasorum, a metastrongylid of canids with very similar biology to that of T. brevior and A. abstrusus. Fluctuations of ecology due to global warming have an effect on the geographical distribution and incidence of these parasites, which is likely due to the cumulative effect of environmental variation on each host and therefore the parasite (Morgan et al, 2009; Traversa, 2014).
The spread of this relatively novel parasite of domestic cats throughout Europe could become a problem of veterinary importance if it continues. This is highlighted by its pathogenicity to kittens, where it can be fatal (Brianti et al, 2012a; Di Cesare et al, 2014). This may be due possibly to its presence in the upper airways (in contrast to A. abstrusus) and its larger size relative to other feline metastrongylids (Brianti et al, 2014). At present, it appears that the parasite is limited to domestic cats of the Mediterranean regions.
Diagnosis and treatment
Diagnosis of respiratory parasitoses is often challenging. Differential diagnoses are pulmonary toxoplasmosis, respiratory mycoses, feline bronchial disease/asthma, airway foreign bodies and pulmonary tumours; treatment with corticosteroids and bronchodilators may produce a clinical recovery in cats, so the veterinarian will not pursue the actual aetiological agent. Features of radiographic examinations are not pathognomonic, the most common findings being an alveolar pattern followed by a bronchial and interstitial pattern, bronchial wall thickening and increased interstitial opacity. No serological assays are available for the diagnosis of feline lungworm.
Therefore, the current antemortem diagnoses are copromicroscopic or airways wash examination that reveal L1s of A. abstrusus and T. brevior and eggs of E. aerophilus. It is known that direct faecal smears and classic sedimentation and flotation methods are unreliable due to random presence of L3, sample size, low sensitivity and larval damage due to the high specific gravity of concentrated solutions. This is why the gold standard technique is the Baermann (larvae migration) test.
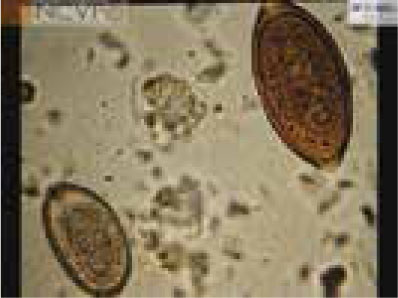
The copromicroscopic examination is the standard test for this diagnosis of E. aerophilus. Note that the eggs of E. aerophilus are very similar to those of Trichuris vulpis (whipworm) (Figure 4); eggs of E. aerophilus have a netlike outer shell, measure ~60–65 x ~25–40 µm, are barrel shaped and have asymmetrical bipolar plugs with no thickening at the base, whereas T. vulpis eggs have symmetrical and ringed plugs and differ in size (Traversa and Di Cesare, 2016).
It is of paramount importance to diagnose the aetiological agent of cardiopulmonary disease in the feline patient to avoid unnecessary dilemmas. Morphological differences are shown in Table 1.
| Larvae | Oral Opening | Tail | |||
|---|---|---|---|---|---|
| Length (mean±SD) | Width (mean±SD) | Length (from the anus to extremity) | Morphology | ||
| Aelurostrongylus astrusus | 360-415 µm (399.1 ± 11.3) | 18-19 µm (18.5 ± 1.2) | Terminal | 40 µm | S-shaped with dorsal and ventral incisures, ventral kink, and evident knob-like protuberance |
| Troglostrongylus brevior | 300-357 µm (338.8 ± 15.6) | 16-19 µm (18.2 ± 1.4) | Subterminal (dorsal) | 35 µm | Same as above but, no ventral kink, and less pronounced knoblike protuberance |
Regarding treatment, in general, protocols for lungworm treatment are straightforward and effective, at least for A. abstrusus and E. aerophilus; efficacy studies against T. brevior are not so easily found.
The treatment options for A. abstrusus and E. aerophilus are:
Broadline spot-on solution for cats (Merial, Lyon, France, recently acquired by Boeringher Ingelheim), which contains fipronil, (S)-methoprene, eprinomectin and praziquantel, was approved in 2013 for the treatment of feline lungworm infestations, targeting A. abstrusus L3 larvae, L4 larvae and adults.
Moxidectin, emodepside, oral milmemycin oxime and spot-on eprinomectin presented favourable data against T. brevior. The first three have been used to treat single cases; eprinomectin proved to be efficacious in stopping larval shedding and also acted against both larvae and adults (Traversa and Di Cesare, 2016).
More field studies are required in mixed infection with these three parasites, taking into account that anthelmintic efficacy against T. brevior depends on age, parasite burden, timing and number of administrations, presence and severity of lung damage and/or host immunity (Traversa and Di Cesare, 2016).
Practical advice and supportive treatment
Lung parasitoses can be difficult to diagnose. Any respiratory sign in a cat at risk, such as a free-roaming domestic cat or kittens, should pop up a warning for veterinary nurses and clinicians working with cats that have travelled from endemic areas, mostly southern Europe, and cats that live in endemic areas. The Baermann migration technique, which the authors believe is not used as frequently as it should be in practice, should be done routinely and a careful assessment of trichurid eggs in the faeces should be carried out.
Mild to moderate cases will normally be resolved by administering anthelmintics. In severe cases, is it critical to control the inflammation and detect concomitant infections, so supportive treatment is needed when the clinical symptoms are severe.
If there are secondary bacterial infections and inflammation, broad-spectrum antibiotics should be administered along with anti-inflammatory doses of corticosteroids, such as oral prednisolone 0.5 mg/kg once a day for 10 days. To promote antibiotic stewardship, these should be selected on the basis of an antibiogram. Doxycycline is one of the best choices when concomitant infections with Bordetella bronchiseptica or Mycoplasma spp. are involved.
If there is respiratory tract congestion, a mucolytic such as bromhexine may help to ease the associated uneasiness and dyspnoea; however, if dyspnoea is severe, then bronchodilators such as theophylline or terbutaline may be useful. In the most severe cases, supportive oxygen administration should be started and, if pleural effusion and pneumothorax are observed, a thoracocentesis is the best option (Elsheikha et al, 2016).
Advice for travelling with cats
The main concern when travelling with domestic cats to endemic areas is to prevent them from roaming too far to prevent consumption of intermediate hosts. Since there is no direct transmission between cats, contact with infected felids poses no direct risk of infection. However, if an area is home to infested cats, this shows larvae at the infective stage are present in the environment.
It is good practice to de-worm and de-flea cats before travelling with them to prevent novel parasite species or strains being introduced to areas where the parasites are not endemic. Conversely, if a cat has not been quarantined after travel (usually if it has come from non-rabies endemic areas), it may be prudent to have a parasite count carried out by a veterinary surgeon or technician and keep the cat indoors until the results are known and any action suggested. This should be done where cats are imported from lungworm endemic regions.
Advice should be sought from a veterinarian when planning to travel with cats to or import cats from endemic regions. Areas of interest because Troglostrongylus is endemic are largely in the Mediterranean regions. However, as the genus spreads through Europe, the geographical distribution of endemicity could change. It may therefore be prudent to consult a veterinarian when travelling to areas in or near to the Mediterranean that have wild or feral cats that could act as a reservoir for feline lungworm.
Conclusion
Lung parasitoses are a major concern in cats, and a proper diagnosis is of paramount importance for the best available treatment. Also, the distinction of mild and severe cases should be made in order to insure appropriate supportive treatment. A close watch on cats while on holiday to endemic areas will aid in the prevention of infection. Veterinary nurses play an important role in the treatment decision and advising clients going on holiday with pets.

