A portosystemic shunt (PSS) is any vascular anomaly that allows blood from the hepatic portal circulation to bypass the liver and be delivered directly into the systemic circulation.
Extrahepatic shunts (EHPSS) are vascular anomalies located outside the hepatic parenchyma. Intrahepatic shunts (IHPSS) are located within the liver. EHPSS may be congenital, usually a single anomalous vessel or acquired, often multiple small vessels. EHPSS account for nearly 63% of single shunts in dogs; they also occur in cats (Fossum, 2007). Acquired extrahepatic shunts (AEHPSS) are typically multiple and represent about 20% of all canine PSS (Fossum 2007). This article will discuss the nursing considerations for patients with EHPSS.
Signalment
Purebred dogs are at increased risk for PSS. EHPSS are most frequently diagnosed in young (under 1 year of age) toy/miniature breeds dogs such as Yorkshire Terrier, Shih Tzu, Lhasa Apso, Maltese and Bichon Frise (Ettinger, 2010).
Presenting signs
Blood bypasses the liver in a PSS, which results in the following:
The reduced portal vein blood flow to the hepatic parenchyma impairs the liver's ability to detoxify digested metabolites. The severity of clinical signs is thought to be related to the volume of blood bypassing the liver. The clinical signs that occur as a consequence of the shunting blood are chronic gastrointestinal signs, lower urinary tract signs, coagulopathies and stunted growth (Berent and Tobias, 2009). The clinical syndrome of hepatic encephalopathy can be associated with altered central nervous system function (Fossum, 2007).
Signs of lower urinary tract disease are common due to decreased hepatic urea production leading to increased urinary ammonia excretion. This predis-poses to the development of ammonium urate crystalluria and urolithiasis. Acute lower urinary tract obstruction may occur as a consequence. Patients may also be polydipsic and polyuric as a result of a poor medullary concentration gradient (Berent and Tobias, 2009). Hepatic encephalopathy and reduced cortisol metabolism may also contribute to polydipsia and polyuria.
Diagnosis imaging
The diagnosis of PSS is confirmed by diagnostic imaging. Methods include:
Definitive diagnosis of PSS is made by surgical identification of the shunt and intraoperative positive contrast portography. This procedure requires laparotomy, fluoroscopy and intravenous contrast material. Once the jejunal or splenic veins are located and catheterised, contrast can be injected into the mesenteric veins which drain into the hepatic portal vein, and the image is captured fluoroscopically. In a normal animal the portal vein branches multiple times into the liver. If there is a PSS, contrast does not enter the liver and is seen to enter the systemic circulation instead. This is the simple most effective portovenography technique (Fossum, 2007).
Laboratory findings
Haematology, serum biochemical and urine analysis of animals with PSS may show variable abnormalities, but dogs can have a congenital PSS without any abnormalities on complete blood count or serum biochemistry panel. Haematology changes include mild to moderate, microcytic, normochromic non-regenerative anaemia.
Serum biochemical abnormalities are extremely common in animals with PSS. In dogs the most common deficiencies include hypoalbuminaemia, decreased blood urea nitrogen, hypocholesterolemia, and hypoglycaemia, which result from decreased hepatic synthesis (Berent and Tobias 2009).
Fasting (12 hours) and 2 hour postprandial serum bile acids are the most widely used tests for evaluating liver function in animals with PSS. Bile acids are synthesised in the liver from cholesterol, conjugated, secreted into the bile canaliculi, and stored in the gallbladder. Bile aids the process of digestion of lipids in the small intestine. Bile acids are then released into the duodenum after a meal, re-absorbed in the ileum, transported into the portal venous system, and extracted by hepatocytes for recirculation. By measuring these levels this entire circuit is evaluated (Berent and Tobias, 2009).
Blood ammonia levels are also increased in animals with PSS due to decreased hepatic ammonia detoxification. Coagulation profiles often show prolonged clotting times as most of the clotting factors are synthesised in the liver (Ettinger, 2010).
Medical vs surgical
The life expectancy of animals that are medically managed is generally reported to be 2 months to 2 years. Fatal liver failure develops after 3 years of age in most dogs that receive only medical treatment, whereas surgical attenuation of shunts is associated with a more favourable long-term prognosis (Frankel et al. 2006). Medical management should be instigated prior to surgery, this usually includes:
These management techniques are an attempt to optimise the patient's condition and reduce the likelihood of post-operative conditions. Surgery is the treatment of choice for most animals with PSS because hepatic function may continue to deteriorate as long as most blood is shunted away from the liver. Patients that are cachectic, encephalopathic or unstable should be managed medically until they can tolerate the stress of anaesthesia and surgery. All pa- tients may benefit on longer medical management to increase body condition score (BCS) prior to surgery.
Anaesthesia
Extreme care should be practiced when anaesthetising a patient with PSS due to:
Fluid and electrolyte imbalances should be corrected prior to surgery. The patient should be starved for no more than 8 hours before surgery due to reduced glycogenolysis and gluconeogenesis reserve.
Anaesthetic agents that are metabolised by the liver, highly protein bound or hepatotoxic should be used with caution. Patients are usually premedicated with opioids and sedatives. Induction agents include propofol or mask delivery of gas (isolflurane/sevoflurane) with oxygen. Patients are then intubated and maintained with a volatile anaesthetic agent (Seymour and Duke-Novakovski 2007) (Table 1).
| Procedure | Used for |
| Peripheral intravenous catheter | Allows the administration of intravenous fuids and induction of anaesthesia |
| Central venous catheter | Allows measurement of CVP during shunt ligation and access to blood sample collection for TP, PCV and blood glucose |
| Arterial catheter | Allow rapid measurement of arterial blood pressure although. Access to blood sample collection for blood gas analysis, TP, PCV and blood glucose |
| Doppler | Technique can be used if at arterial catheter cannot be placed. |
| ECG | To monitor heart rate and rhythm during surgery |
| SPO2 | To monitor oxygen saturation during surgery |
| etCO2 | To monitor end tidal C02 during surgery |
Patients with PSS are often hypersensitive even at light levels of anaesthesia and therefore a balanced anaesthetic technique is advantageous. Hypoglycaemia can result due to impaired ability of the liver to synthesise glucose and reduced hepatic glycogen stores. Therefore, blood glucose levels should be monitored throughout anaesthesia.
Care should be taken to prevent hypothermia as these patients are often small and they have a large surface area:volume ratio and readily lose core body heat due to open abdominal cavity and the surgical preparation solution used. Body temperature can be maintained by using warm blankets, hot air blankets and bubble wrapping extremities.
Surgical preparation and the veterinary nurse's role
The veterinary nurse has a vital role in contributing to the success of this procedure by preparing equipment and theatre considerations for the surgery to run as smoothly as possible.
It is ideal that the veterinary nurse has a good idea of the surgical technique as they might be required to scrub in and assist with the procedure. The nurse can prepare the surgical table and anticipate the required instruments, retract, lavage, observe for any portal pressure changes and prepare contrast equipment.
Surgery options
The preferred treatment for PSS is complete/partial shunt attenuation. Surgical attenuation is intended to redirect the portal blood flow through the liver to promote normalisation of the hepatic structure and function (Lee et al, 2006).
An ideal method of surgical treatment would be a single quick, inexpensive procedure that resulted in gradual attenuation without the risk of portal venous hypertension. Most will not tolerate complete occlusion of shunts in one procedure therefore gradual attenuation is preferred to reduce the risk of post-operative complication. Successful surgical attenuation of shunts can lead to a complete clinical recovery without the need for long-term medical or dietary treatment.
Once the anomalous vessel is identified it will be temporarily occluded with plastic tubing or/ and nylon tape, then portovenogram performed to ensure the correct vessel is occluded (Figure 3). The surgery can be continued with the attenuation method of the surgeon's preference. There are several methods of attenuation:
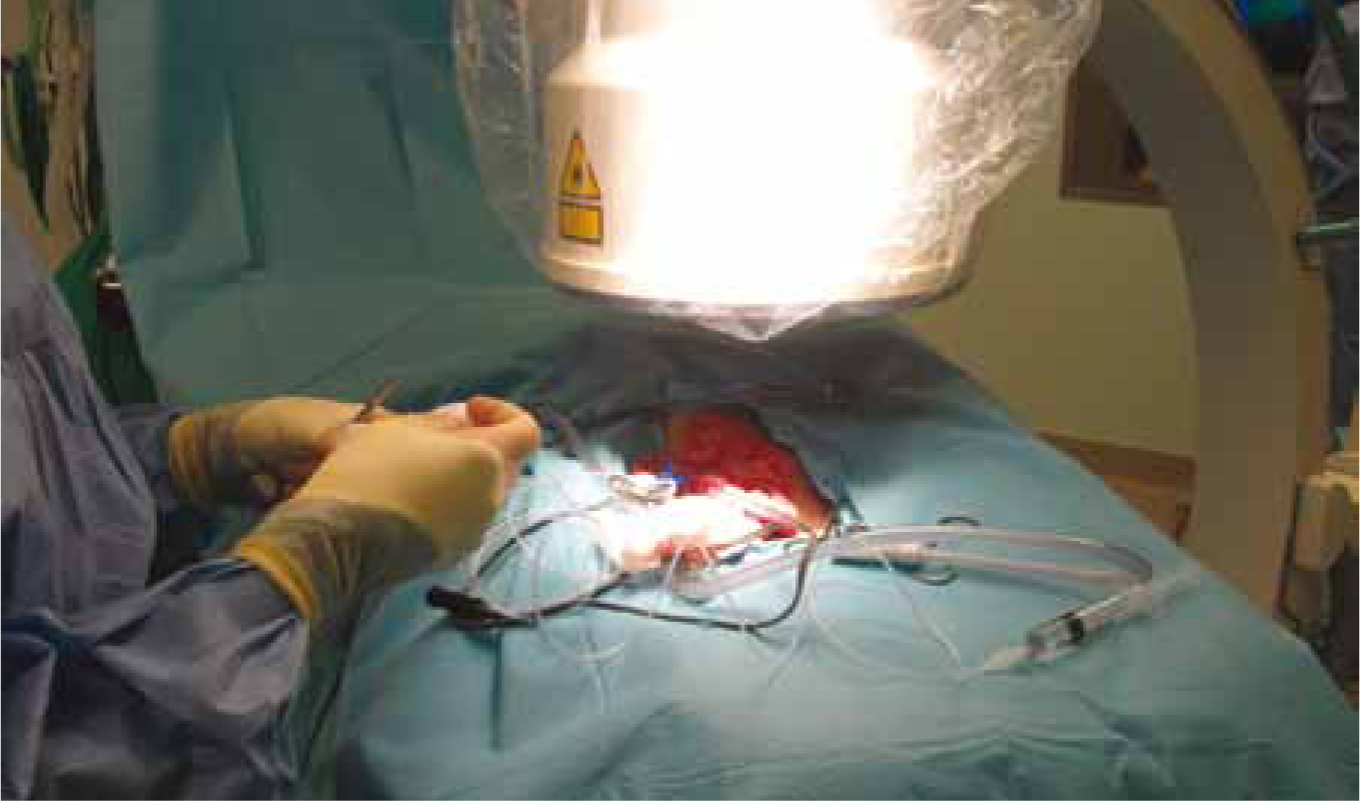
Ameroid constrictors and cellophane bands are the most common attenuation techniques used as the suture technique requires a second procedure. Ameroids are preferred physiologically as the liver has a chance to adapt to blood flow. They are less likely to cause portal hypertension making them a convenient option, whereby less monitoring is required (Fossum, 2007).
Pre and post-ligation portograms are used to assess the portohepatic blood supply in animals undergoing suture attenuation of PSS. Acute complications for the nurse to observe include:
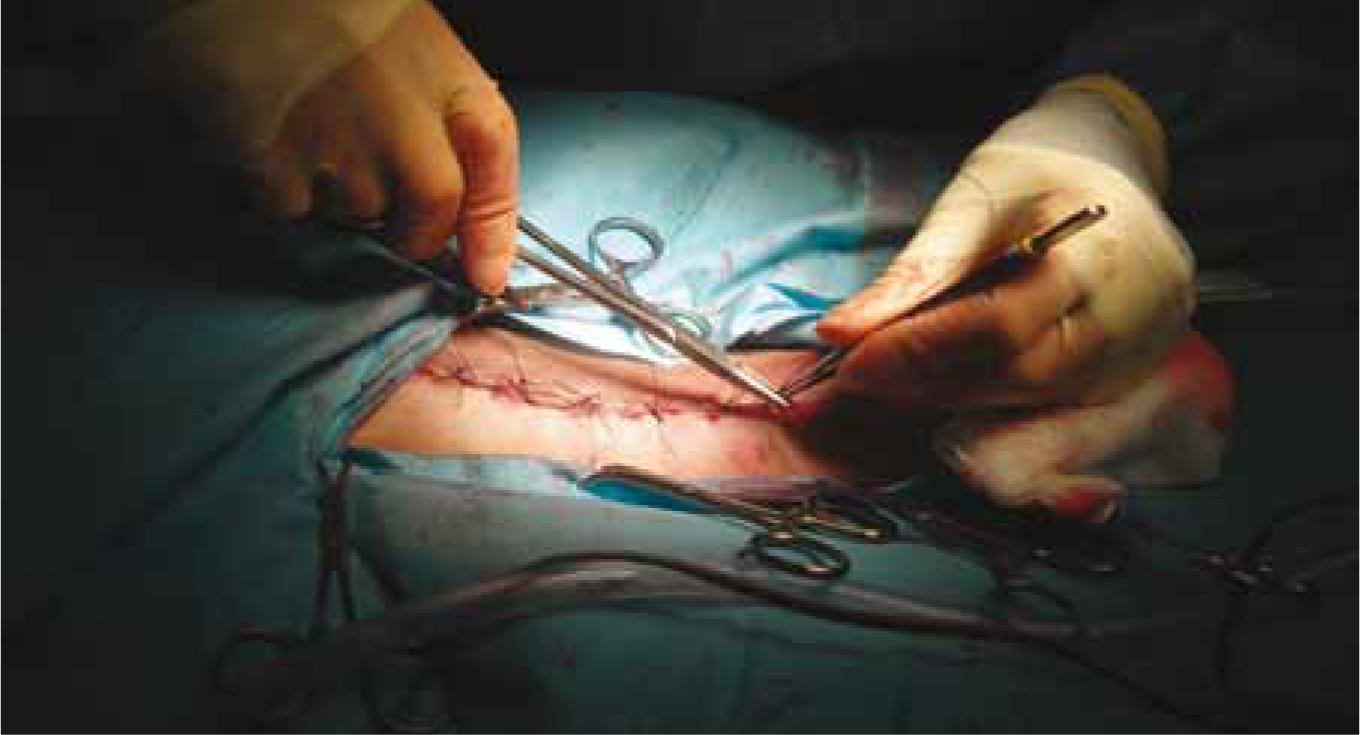
Before abdominal closure, the surgeon should perform a liver biopsy to rule out other hepatic diseases once the shunt is attenuated (Figure 4). The bladder is often palpated for calculi and these are removed via cystotomy. Swabs are counted.
Post-operative nursing care
For the purpose of this article the post-operative period is defined as the time from anaesthesia recovery to 72 hours post operatively. PSS patients are often hospitalised within the intensive care unit for 72 hours post surgery as this time period is associated with a high risk of post-operative complications such as transient ascites +/- wound dehiscence, mild self-limiting vomiting and diarrhoea, surgical wound infection, cystitis secondary to cystotomy, septic peritonitis, seizures and severe blood loss and anaemia (Lee, 2006).
Post general anaesthesia
Hypothermia during recovery is a common problem and can be minimised by recovering patients in an incubator. Active surface warming increases the air temperature around the patient, therefore increasing the patient's temperature (Davies, 2012). Other warming options include Bair Huggers, heat mats and wheat bags. If an incubator is used, flow by oxygen can be administered as shivering patients have a higher oxygen demand than those that are not shivering.
Blood pressure should be monitored every 15-30 minutes. These patients are often hypotensive (mean arterial pressure <60 mmHg). Colloids can be given if the patient is hypotensive and does not respond to crystalloids. Fluid therapy should be continued until the patient is eating and drinking. The rate of fluid therapy will be reduced from the surgical rate to a maintenance rate during recovery if the central veouns pressure (CVP) readings are within the normal limit (0-5 cmH20).
Blood glucose should be monitored hourly until the patient is awake.
Due to reduced liver function drugs may be metabolised more slowly than normal and so dosing intervals and/or amounts may need to be adjusted. Methadone is often given at a dose of 0.1-0.3 mg/kg intravenously. This can be increased or decreased as necessary. The patient should be pain scored hourly using the Glasgow Composite Pain Score (Murrell, 2008). If a score of 4 or more is demonstrated then the analgesia plan should be reevaluated. Non-steroidal anti-inflammatory drugs should be avoided if possible due to the risk of GI ulceration associated with portal hypertension.
General nursing care should be provided to meet the patient's individual needs such as turning recumbent patients every 2 hours, lubricating eyes and ensuring the head and neck are extended once extubated.
First 24 hours
Vital signs should be monitored every 2-4 hours. This includes pulse rate, respiratory rate, mucous membranes and capillary refill time, blood pressure and temperature (until stable).
The analgesia plan can be reevaluated depending on the pain score. Analgesia can be staged down from methadone to buprenorphine. The patient should be pain scored when the analgesia is expected to wear off. When a score of 4 or more is determined the next dose of analgesia should be given.
An in-house packed cell volume (PCV)/total solids, electrolytes and acid-base balance should be performed daily. Electrolyte and acid-base abnormalities should be highlighted to the case veterinary surgeon and addressed as necessary (i.e. treating hypokalaemic patients with potassium chloride supplementation).
The central venous catheter is usually kept in situ for the first 24 hours or until the patient is eating and drinking with enthusiasm. The central line should be checked twice daily for signs of infection, inflammation or migration. It should be dressed with a transparent semi-permeable dressing over the skin/ catheter site and then a light neck bandage. If there is discharge from the skin site then the dressing should be removed, the site cleaned with dilute chlorhexi-dine and a sterile dressing applied. The strictest level of hygiene must be adhered to with the use of gloves to handle the ports and rebandaging.
Mentation should be monitored every 4 hours. The patient should be bright, alert and responsive. They should interact with the surroundings. If mentation is altered then there may be concern that the patient is becoming encephalopathic due to elevated ammonia within the bloodstream. This can be checked via a laboratory test and the patient can be assessed using the Modified Glasgow Coma Score (Platt, 2009). Treatment for hepatic encephalopathy is often aggressive and requires lactulose enemas, oral lactu-lose and a switch from oral antibiotics to intravenous preparations. In severe cases the patient may seizure and require anticonvulsants such as levitaracetam and even induction of a propofol coma. As a standard policy each PSS patient at the authors' practice is issued with a seizure sheet which indicates the course of treatment required should the patient seizure. This includes the order and doses of drugs to be given by the veterinary nurse in an emergency.
The abdominal circumference of the patient can be measured with a tape measure to monitor for portal hypertension. This is less likely to develop in patients with a gradual method of occlusion such as an ameroid constrictor and more important if suture ligation is used. This occurs when the liver is unable to cope with the increase in blood supply once the shunt has been closed. Venous congestion then develops in organs normally drained by the portal system. Signs of portal hypertension include ascites, pain, melaena and systemic hypotension (Silverstein and Hopper, 2009).
Most patients will already be on a protein restricted diet. Ideally the owner should provide some of the patient's usual diet for while they are hospitalised. As these patients are often small and prone to hypoglycaemia, it is important to encourage eating as soon as possible. The authors believe that if the patient is averse to eating a low protein diet then they should be tempted with something else until they are eating. Food should be offered every 2-4 hours.
The medical management that the patient was receiving prior to surgery should be continued. This often consists of oral lactulose and amoxicillin or ampicillin. This should help reduce the risk of hepatic encephalopathy.
Patients with liver disease can have coagulation disorders, and so they should be carefully monitored for signs of bleeding, particularly if liver biopsies have been taken. Signs of a developing haemoabdomen include depression, tachycardia, hypotension, pale mucous membranes and abdominal distension. If there are concerns over haemorrhage, a body bandage can be applied and the patient will require cardiovascular stabilisation.
The surgical wound should have a light adhesive dressing applied for the first 24 hours until a fibrin seal has formed. If there is strike through to the dressing then it should be replaced.
Urine output should be monitored by noting on the hospitalisation record when the patient voids their bladder. Normal urine output should be 1-2 ml/kg/ hour. It is important to be aware of ‘ins and outs’ as the absence of urination may indicate acute kidney injury.
48-72 hours
If the patient is eating and drinking then intravenous fluid therapy can be discontinued. If the patient is comfortable on the pain score then analgesia can be terminated.
The surgical wound should be monitored daily for signs of inflammation or infection. The central line can be removed if it has not been yet. At this point the patient should be fit for discharge or if hospitalisation is required until the owners can collect their pet then they can be hospitalised in a normal ward area. Medical management is usually continued for at least 4 weeks post operatively when repeat laboratory tests will be scheduled.
Conclusion
Surgical intervention is the best treatment option for EHPSS patients. Nursing such patients is a challenge for any experienced nurse due to the individual patient requirements and the continuous planning, implementation, assessment and evaluation. Such cases require a team approach and can be very rewarding.
