Anoxious stimulus activates the body's normal sensory system and there are certain stages identified in the pain pathway; these are transduction, transmission, modulation and perception. There are two types of fibres associated with the transmission of pain: A delta fibres are large in diameter and myelinated, and produce sharp or well-defined pain to a noxious stimulus so fast the body responds immediately; smaller C fibres transmit dull or aching pain, and transmit the pain signal slower than A delta fibres due to their lack of a myelin sheath (Helms and Barone, 2008).
Nociceptors, or primary afferent neurons are activated due to tissue injury caused by mechanical, chemical or thermal sources, and are found in skin, muscles, joints, connective tissue (somatic tissues) and some visceral tissues. Transduction (the first stage in the pain process) occurs when nociceptors are exposed to a sufficient stimulus to cause depolarization of the nerves, and transmission (the second stage) of this impulse generated occurs along the axon of the neuron, which continues to the spinal cord and higher centres (Benson, 2000). Perception is the third stage, where the cerebral cortex receives the signals from the peripheral and spinal nerves in order to formulate a response. Modulation is the final stage of the pain process, where the afferent neurons release substances that expand the nociceptive field to areas adjacent to the original injury; this is essentially the start of ‘wind up’, or secondary hyperalgesia (Figure 1).
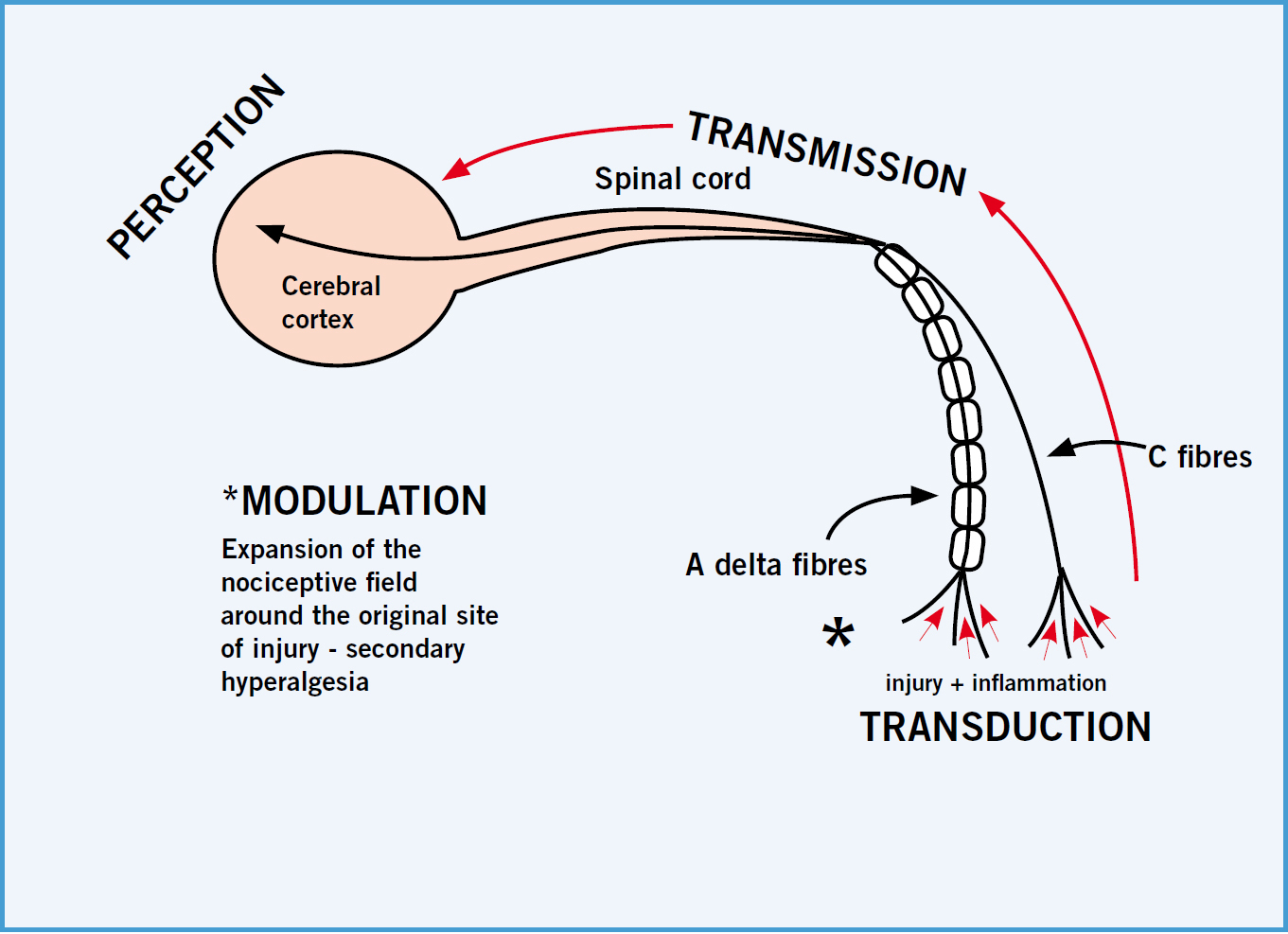
The pain experience from somatic and visceral tissues can be acute or chronic in nature (Helm and Barone, 2008), and somatic and visceral pain can be further differentiated. Pain caused by the ongoing or prolonged activation of nociceptors innervating bones, joints, muscles and connective tissues is termed somatic pain and tends to be well localized to a specific site of injury. Somatic pain has been described (by humans) using adjectives such as aching, throbbing, stabbing and squeezing. Visceral pain is caused by the activation of nociceptors that innervate visceral structures, which is typically difficult to localize and varies in intensity, a waxing and waning type of pain. Humans have described this type of pain as cramping or gnawing, and an example would be the pain caused by the obstruction of a hollow organ (American Medical Association, 2007).
Acute pain, if not detected early or managed well, can cause peripheral neural wind up, which then makes pain harder to control and manage in patients, even promoting the development of chronic pain (Clark, 2010). Neurogenic inflammation causes the release of various substances from the nerve endings, such as serotonin, histamine, acetylcholine and bradykinin, which serve to activate and sensitize other nociceptors; this is primary hyperalgesia. This, combined with the effects of prostaglandins from damaged tissues, enhances the nociceptive response to inflammation, thus lowering the patient's threshold to noxious stimuli (Hudspith et al, 2012).
Preventing and controlling pain should be central to veterinary medicine (Benson, 2000) and classed as the fourth vital sign after temperature, pulse and respiration. Through understanding the mechanisms associated with pain veterinary professionals can anticipate how it might occur, when it might occur, how it might progress, and thus plan to ideally prevent it or manage it well (Hellyer et al, 2007).
Pain in critically ill animals
There is a growing body of evidence in human medicine regarding pain management in critically ill people, and this supports the notion that effective pain management in humans decreases morbidity and mortality (Fagella, 1997). If this is true for humans, there is no reason why it should not be considered true for animals. Pain, unfortunately, is often undertreated in critically ill animals, and Fagella (1997) suggested the reasons why include:
- Veterinarians being worried about contributing to the cardiopulmonary instability of the patient
- The perceived difficulties in small animal practice surrounding the monitoring of patients' responses to the analgesic therapies provided.
These factors should not stop veterinarians providing critically ill patients with effective and appropriate analgesics as they can definitely improve their clinical statuses, so their use or non-use could ultimately improve or hinder their recovery. Table 1 outlines the frequently overlooked causes of pain in dogs and cats, which all veterinary nurses (VNs) must be aware of, as described by Hellyer et al (2007). This table highlights the scope and range of ways in which pain can be underestimated in patients, and for critically ill patients this is even more of a problem because they tend to require more invasive interventions to treat their conditions.
Table 1. Frequently overlooked causes of pain
| Type of pain | Cause |
|---|---|
| Cardiopulmonary | Congestive heart failure (pulmonary oedema, pleural effusion); pleuritis; cerebral vascular accident (CVA); thromboembolism |
| Oncologic | Any and all cancer |
| Dermatologic | Otitis; severe pruritus; burns; chronic wounds; abscess; cellulitis; clipper burns; urine scalding; severe chin acne |
| Dental | Oral tumours; feline odontoclastic resorptive lesions (FORL); fractures; tooth abscesses; ulcers (Figure 2); stomatitis |
| Gastrointestinal | Constipation; obstipation; obstruction; megacolon; anal sac impaction; haemorrhagic gastroenteritis; pancreatitis; gastric dilatation-volvulus (GDV); foreign body |
| Musculoskeletal | Most often overlooked in cats. Muscular soreness; arthritis; degenerative joint disease; tendon or ligament injury; intervertebral disc disease; facet pain of spondylosis; osteodystrophy; dislocations |
| Ocular | Corneal disease; corneal ulcers; glaucoma; uveitis |
| Urogenital | Uroliths; ureteroliths; queening/whelping; feline lower urinary tract disease/interstitial cystitis; acute renal failure; enlarged kidneys; lower urinary tract infections; urinary obstructions; vaginitis |
| Hospital procedures | Restraint (examination, obtaining blood and urine samples, radiographs and ultrasound — gentle handling on a hard surface can increase pain in an already painful animal); urinary and intravenous catheterization; bandaging; surgery; thoracocentesis; chest tube placement and drainage procedures; abdominocentesis; manual extraction of stools and anal gland expression |
| Surgical procedures | Ovariohysterectomy; castration; onychectomy; growth removal and all other surgical procedures |
| Neurologic | Diabetic neuropathy |
Consider a patient presenting in respiratory distress due to a pleural effusion. As well as the immediate problem of being unable to adequately oxygenate its tissues and associated cardiopulmonary pain, the patient is likely to suffer all of the following causes of pain in the course of its treatment:
- Potentially clipper burn (thoracic wall clipping and clipping for intravenous (IV) catheter placement)
- Depending on the age, it may already have a degree of musculoskeletal pain that could be exacerbated by handling and restraint
- IV catheterization
- Thoracocentesis
- Chest tube placement if the pleural effusion reoccurs (unilateral or bilateral)
- Drainage of the chest tubes as required.
The effects of pain
As discussed by Quandt et al (2005), critically ill patients will benefit from an effective analgesic treatment plan because pain that is left untreated can have detrimental effects on:
- Healing
- Nutrition
- Immune system
- Overall wellbeing.
Pain provokes a stress response in patients causing them to release corticosteroids from their adrenal glands, which can delay healing if it is a prolonged response. Pain is depressing for animals and suppresses their appetite and activity. Pain can also impair normal respiratory function causing a reluctance to cough, which is a natural respiratory defence mechanism in mammals.
Gogny (2006) further highlighted some of the morbid effects of pain that must be considered in critically ill patients, which are mainly associated with the neuroendocrine disturbances, including changes in:
- Cardiac output
- Vasomotor responses
- Fluid and electrolyte balance
- Phosphorus and calcium balance.
Acute, severe pain can lead to cardiogenic shock whether the patient is conscious or not, and the increased heart rate and after-load increases myocardial oxygen consumption, which can result in the development of arrhythmias.
The assessment of pain and provision of appropriate analgesia should always be part of the treatment plan for critically ill patients, and veterinary professionals are becoming more aware of the importance of this aspect of care. It is obvious to all that injured and surgical patients require analgesia, but people sometimes forget the patients that are most critically ill in an intensive care unit (ICU), and that require the most aggressive analgesic regimen possible.
The assessment of pain
Assessing pain in animals is often difficult as they are all individuals with differing natures, experiences of pain, clinical conditions and pain thresholds. Animals cannot self report on their pain experiences as is gold standard in human patients, thus pain can only ever be assessed in animals according to how their pain is perceived (Coleman, 2006;Adamantos, 2008). There are numerous methods and published tested pain scoring models available for use, but the veterinary profession is still trying to create an ideal pain scoring system for animals; it will always be a struggle to objectively quantify a phenomenon which is subjective in nature in patients that cannot express where it hurts (Johnson, 2007). Table 2 highlights some of the ways in which patients might express their pain both visually/behaviourally and physiologically.
Table 2. Ways in which patients might express pain
| Visual/behavioural | Physiological |
|---|---|
| Vocalization | Tachycardia |
| Trembling | Tachypnoea |
| Protecting painful areas | Salivation |
| Reluctance to move | Hyperglycaemia |
| Lameness | Elevated serum cortisol/corticotrophin |
| Atypical behaviour (must know what is normal to assess this well) | Hypertension |
| Anorexia | Mydriasis |
| Sleep deprivation | |
| Lack of grooming | |
| Fear/anxiety/apprehension/aggressiveness | |
| Licking/mutilating the painful area |
The VN must be vigilant when assessing critically ill animals to identify any of the changes that might indicate pain. Often there will be a number of these changes evident in one patient, which provides more evidence that a patient is definitely in pain and requires treatment. If a very detailed hospitalization sheet is being used, or a nursing care plan which considers and assesses many of the patient's activities or abilities of daily living, it will be easy to identify a painful animal from a comfortable one.
From a holistic nursing perspective it is clear that pain has a detrimental effect on many aspects of a patient's life: the ability to groom; the ability to be mobile; the ability to eliminate; the ability to eat and drink; the ability to rest and sleep; the ability to express normal behaviour, and so on. Thus, the more painful a patient is or the longer it suffers without analgesic intervention, the more numerous, complex and frequent the nursing interventions are going to be.
Once identified and an appropriate analgesic regimen formulated by the veterinarian, it is the VN's duty to monitor the effects of the treatment. This involves the VN reassessing everything mentioned in Table 2, accurately recording physiological parameters and identifying trends or patterns in the data. It may be useful for the VN to use a pain scale in an attempt to quantify and monitor pain in animals, and the most common ones available for use are summarized in Table 3.
Table 3. Common pain scales
| Pain scoring scale/system | Comments |
|---|---|
| Simple Descriptive Scale (SDS) | Original for human use |
| Numerical Rating Scale (NRS) | Original for human use |
| Visual Analogue Scale (VAS) | Original for human use |
| Variable Rating Scale (VRS) | All have been used successfully in veterinary patients |
| Dynamic and Interactive Visual Assessment Scale (DIVAS) | |
| Glasgow Composite Pain Scale (GCPS) | |
| Melbourne Pain Scale (MPS) |
Patient comfort in relation to critically ill patients is of paramount importance to their survival, and monitoring of their pain status alongside their organ function, hydration status, electrolyte balance, nutrition, body temperature control, infection monitoring, recumbency care and haematological/biochemical status is essential (Bilbrough, 2003).
Multimodal analgesia options
Hughes (2008) indicated that there are many different analgesic drugs available for use in veterinary practice nowadays including non-steroidal anti-inflammatory drugs (NSAIDs), local anaesthetics and opioids, of which the latter tends to be most widely used (see Table 4 for commonly used analgesic drugs for critically ill patients). Multimodal, or polymodal analgesic regimens should be devised, implemented and monitored carefully for all critically ill or injured animals, and the variety of drugs and therapies available for use in practice can interrupt the pain pathway at different stages:
- Inhibit transmission — local anaesthetics, alpha 2 agonists
- Inhibit transduction — NSAIDs, opioids, local anaesthetics
- Modulate spinal pathways — local anaesthetics, alpha 2 agonists, NSAIDs, NMDA antagonists
- Inhibit perception — opioids, alpha 2 agonists.
Table 4. Commonly used analgesic drugs for critically ill patients
| Drug | Group | Comments |
|---|---|---|
| Meloxicam | NSAID | NSAIDs do provide very good analgesia for veterinary patients, but the toxic side effects are more likely to affect critically ill/trauma patients. They should not be used until a patient is fluid resuscitated and has had its renal and gastrointestinal functioning thoroughly assessed. The doses and dosing intervals are usually different in cats than dogs |
| Carprofen | NSAID | |
| Butorphanol | Partial mu agonist | Duration of action is around 90 minutes, like pethidine, but it provides more sedation and less analgesia. It acts as an antagonist at mu receptors so should not be used in combination with pure mu agonists (it will reverse their effects) |
| Buprenorphine | Partial mu agonist | 30 minutes' onset but lasts 6–8 hours. Generally a very good analgesic, but will also act as an antagonist at mu receptors like butorphanol |
| Morphine | Pure mu agonist | Potent analgesic can be given via IM, IV, CRI, epidural, intra-articular and ocular routes. In healthy, pain-free patients it can cause vomiting and bradycardia |
| Methadone | Pure mu agonist | Has the same analgesic potency as morphine, but produces less sedation and has a long duration of action in dogs |
| Pethidine | Pure mu agonist | Short-acting opioid — duration 90 minutes with a rapid onset of 10 minutes or less. It generally does not induce vomiting and causes less sedation than morphine. This should not be given IV as can cause a release of histamine |
| Papaveretum | Pure mu agonist | Contains all four major alkaloids of opium, and is a long-established drug. It provides a better degree of sedation than morphine and has fewer gastrointestinal side effects |
| Fentanyl | Pure mu agonist | Rapid onset of 2–3 minutes, and duration of action is approximately 30 minutes. Can be used as ‘rescue analgesia’. High doses and CRI administration can cause respiratory depression. Transdermal patches are most useful |
| Lidocaine/bupivicaine | Local anaesthetics | Completely block nociception. Can be used for epidural analgesia, intercostal nerve blocks, intrapleurally via chest drains, into wound catheters and as splash blocks on wounds/fractures. Cats are more sensitive to the toxic side effects though, so avoid using in them |
IM, intramuscular; IV, intravenous; CRI, constant rate infusion
In addition to NSAIDs, local anaesthetics and opioids there are other drugs which have useful analgesic properties for critically ill patients, including:
- Ketamine — an NMDA antagonist
- Medetomidine — an alpha 2 adrenergic agonist
- Tramadol and gabapentin — neuropathic pain
- Tricyclic antidepressants.
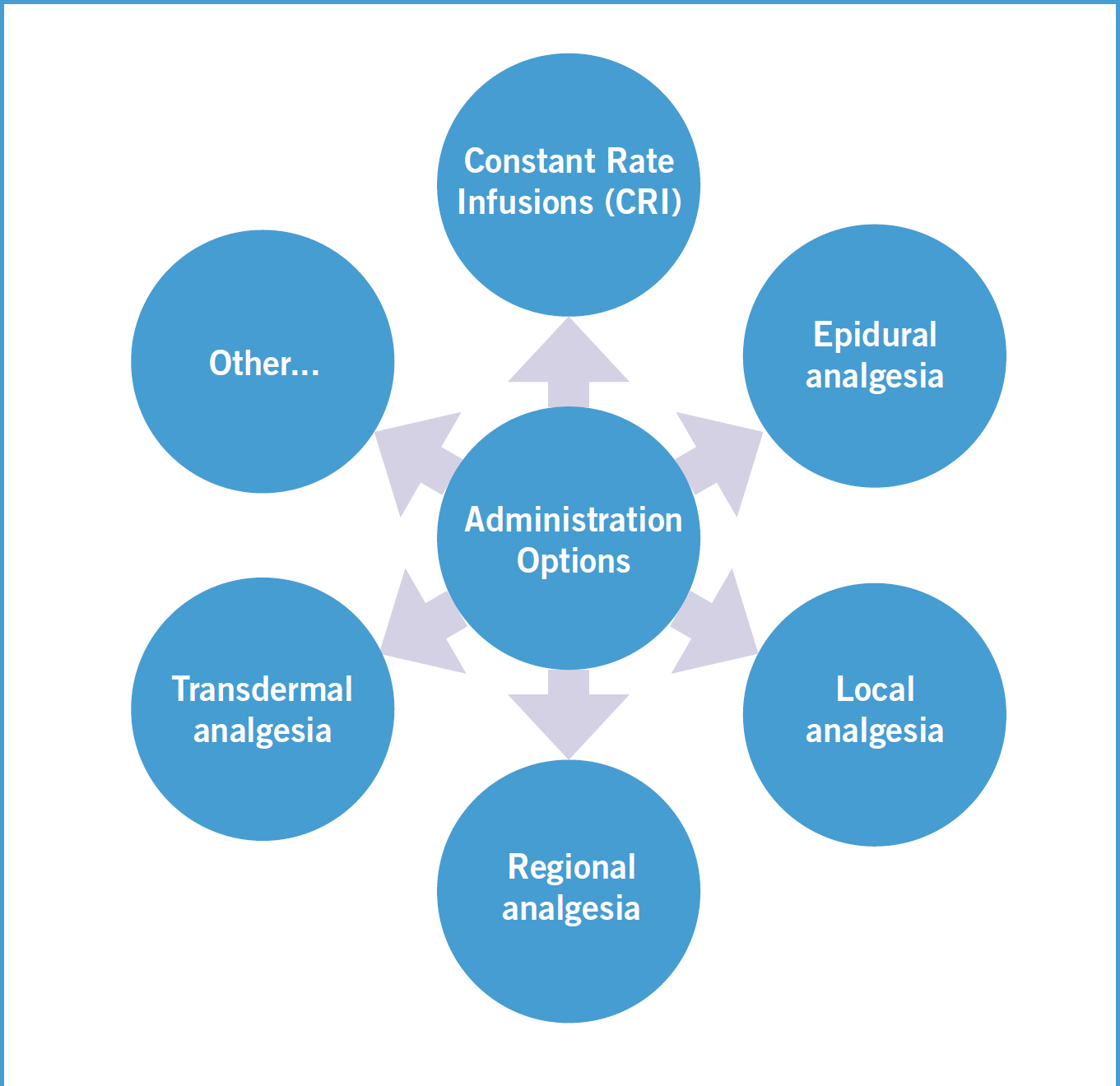
There are also other options for analgesic administration apart from periodic dosing (see Figure 1 for alternative administration options). Considering the administration options in Figure 3, here is a little more detail regarding these options for critically ill patient analgesia, as outlined by Hughes (2008):
- Morphine constant rate infusion (CRI) — provides analgesia with sedation after major surgery or trauma.
- Ketamine CRI — as an NMDA antagonist it can block central sensitization and wind up. It is good for patients with chronic pain and those failing to respond to initial or more traditional analgesic regimens. It can increase intra-cranial pressure though so should be avoided in cases of head trauma.
- Lidocaine CRI — low dose CRI use can augment opioid analgesic use in dogs and cause mild sedation. They are therefore good in patients that require high doses of opioids, as the dose of the latter can be reduced thus reducing the potential side effects. Does not usually cause cardiovascular depression but side effects can be seen with prolonged use.
- Medetomidine CRI — low doses provide very good analgesia as an adjunct to other techniques.
- Morphine, lidocaine and ketamine (MLK)CRI — MLK mixed together in saline (in specific ratios) provides excellent analgesia with sedation and can be titrated to the patient's needs. Can help provide high quality multimodal pain control in small animals (Mason, 2009).
- Morphine epidural — lumbo-sacral epidural injection can provide 12 to 22 hours of analgesia to the pelvic limbs, abdomen and sometimes the thorax. This does not cause motor nerve blockade like local anaesthetics would. Rather than repeated epidural injections an epidural catheter can be placed, and if well maintained remain in place for many days for intermittent (slow) injections of morphine or for CRI administration (Hanson, 2001). Injecting a small volume of lidocaine prior to the morphine has been found to reduce the chance of vomition.
- Fentanyl transdermal patches — there are 25, 50, 75 and 100 microgram per hour patches available for veterinary use, which deliver these doses for 72 hours. It takes 24 hours in dogs and 12 hours in cats to reach peak plasma levels, so until that point is reached, other analgesia must be provided. They are also typically used as part of a multimodal analgesic regimen, i.e. not as the sole form of analgesia. The patches should be applied to clipped skin, covered with an occlusive dressing, not heated (i.e. by a heat pad as it enhances the dose delivered) and disposed of correctly as there is potential for abuse.
- Other — other types of nursing interventions that form a part of a multimodal approach to analgesic provision include: stabilizing fractures; emptying a patient's full bladder; providing a comfortable bed; ensuring a patient's bed is always clean and dry; ensuring the patient receives adequate nutrition; grooming a patient and maintaining a healthy, clean coat; interaction with caregivers (if that is what the patient likes — some are happier with no contact); and the ever important tender, loving care (TLC). Sedation can be useful if a patient is not fully settling having been administered analgesics, using drugs such as acepromazine or benzodiazepines (midazolam/diazepam).
Conclusion
Veterinary professionals must not allow their worries about the side effects of different drugs to prevent their use; they should research current thinking about the wide variety of drugs and therapies available, assess each patient individually and thus create the most appropriate and tailored regimen for that patient for their optimal comfort and potential recovery.
Key Points
- Veterinary professionals must understand the physiology of pain, the effects of pain and the consequences of improper management of pain.
- When monitoring critically ill patients, veterinary nurses must be aware of the variety of signs of pain exhibited by animals and also be mindful of the commonly overlooked sources of pain.
- A multimodal, or polymodal approach to analgesia provision in critically ill patients is essential to their comfort and wellbeing, and it must not be forgotten that many nursing interventions contribute to analgesia, not just drugs.
To answer the CPD questions on this article visit www.theveterinarynurse.com and enter your own, personal login.The Veterinary Nurse CPD is approved by Harper Adams University College

