As veterinary medicine is continuously evolving, so are the expectations for the level of patient care. In the veterinary emergency and critical care setting, patient care checklists can be utilised to optimise patient care quality and standards. Checklists create a step-by-step process of evidence-based interventions and procedures that help prevent medical oversights (Berenholtz et al, 2002; Fulbrook and Mooney, 2003).
Kirby's Rule of 20 is a checklist created by Rebecca Kirby, DVM, DACVIM, DACVECC that includes 20 patient parameters that should be evaluated daily in critically ill patients. Kirby's Rule of 20 was created as a reminder for veterinarians and veterinary nurses to examine the status of critically ill patients, including organ systems involved, clinical parameters, diagnostic parameters, and treatment goals in order to optimise patient survival (Kirby, 2017). Following the Kirby's Rule of 20 checklist allows veterinary nurses to assess the overall clinical picture of a patient (taking a holistic approach), implement critical thinking skills, elevate the quality of patient care, set standards for patient care, and decrease morbidity and mortality which results in improved patient outcomes (Berenholtz et al, 2002; Fulbrook and Mooney, 2003).
The patient parameters in the Kirby's Rule of 20 checklist are:
- Fluid balance
- Albumin and oncotic pull
- Electrolyte and acid–base
- Mentation
- Heart rate, rhythm and contractility
- Blood pressure
- Body temperature
- Oxygenation and ventilation
- Red blood cells (RBCs) and haemoglobin
- Coagulation cascade
- Renal function
- Gastrointestinal (GI) motility and integrity
- Nutrition
- Glucose
- Immune status and antibiotics
- Wound healing and bandages
- Drug dosage and metabolism
- Pain control
- Nursing care
- Tender loving care.
To allow enough detail for each parameter, part 3 will focus on the physiology, clinical application, and monitoring of patient parameters 11–15.
Renal Function
The renal system (comprising the kidney, ureters, bladder, and urethra) is responsible for many functions that help maintain overall homeostasis. Its roles include fluid regulation, hormone production, and excretion of metabolic waste products (Scalf, 2014). The kidneys maintain the volume and composition of body fluids (water and electrolyte balance), absorb solutes (proteins, amino acids, glucose), remove metabolic waste from the body (urea, uric acid, creatinine), and receive approximately 20–25% of overall cardiac output to help maintain arterial blood pressure (Smarick and Hallowell, 2015; Steele, 2019). When any of the kidney's functions become disrupted, there can be systemic consequences.
In critically ill patients, acute kidney injury (AKI) is a primary concern because of the various disease processes that can result in renal function disruption. AKI is defined as the abrupt (acute) inability of the kidney to regulate fluid balance, electrolyte balance, acid–base balance, and the ability to excrete body waste products. AKI has been classically categorised based on the types of azotaemia that results from the underlying disease process. Azotaemia is recognised by abnormally high concentrations of body waste compounds within the blood, primarily blood urea nitrogen (BUN) and creatinine. Azotaemia resulting from AKI can further be grouped as prerenal, intrinsic renal, or postrenal, and is reflective of the origin of disease (Herold, 2017). Prerenal refers to ‘before’ the kidney, meaning injury is caused by non-renal physiological or haemodynamic factors in which renal perfusion is compromised, affecting renal blood flow and causing ischaemic injury. Examples include hypovolaemia, dehydration, cardiac compromise, or vasodilatory diseases. Intrinsic renal refers to direct damage to the renal parenchyma. Examples include renal ischaemia, exposure to toxic agents, or infectious insult. Postrenal refers to ‘after’ the kidney, meaning there is an obstruction or impediment in the outflow of urine that prevents urine from being eliminated from the body. Examples include urethral obstruction, strictures, prostatic disease, urolithiasis, inflammation, trauma, and neoplasia (Langston and Eatroff, 2015; Steele, 2019).
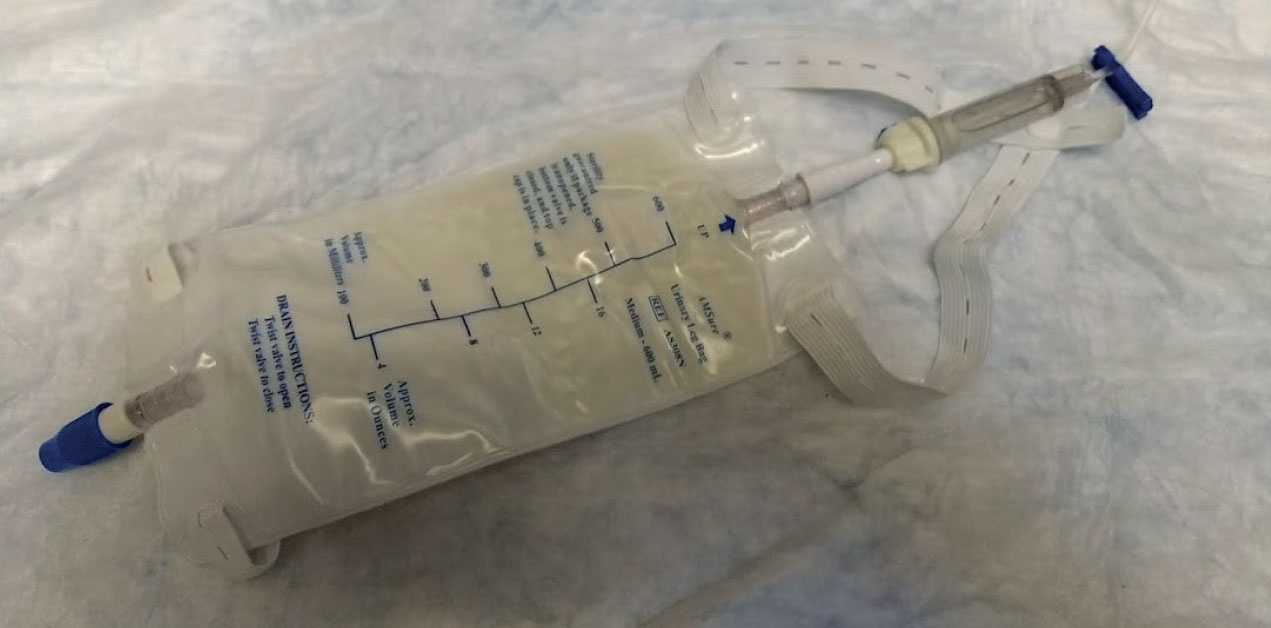
To ensure adequate monitoring of the renal system, a variety of parameters are assessed, including patient parameters, fluid balance, urine output, and laboratory values (BUN, creatinine) (Herold, 2017). Routine assessment of mentation, heart rate, pulse rate, respiratory rate, temperature, mucous membrane colour, capillary refill time, and blood pressure are all important patient parameters because they are related to adequate cardiac output and renal perfusion (Langston and Eatroff, 2015). AKI patients are more prone to fluid complications as a result of a compromised renal function, and therefore require assessment of fluid balance. Fluid balance involves careful monitoring of the amount of fluids in (intravenous (IV) crystalloids, IV medications, oral intake), amount of fluids out (urine output), and bodyweight (fluid retention versus fluid loss) (Herold, 2017). Urine output (UOP) is an outward indicator of appropriate renal function (Smarick and Hallowell, 2015). As a reference, normal UOP in a well-perfused, hydrated animal should be between 1–2 ml/kg/hour (Smarick and Hallowell, 2015). Methods of monitoring UOP include collecting and measuring voided samples, weighing kennel bedding or litterboxes (1 g = 1 ml), or placing a urinary catheter (Aldrich, 2012) (Figure 1). Laboratory values that should be evaluated include serum biochemistry (BUN, creatinine), packed cell volume/total protein (PCV/TP) (hydration status), and urinalysis (urine specific gravity, urine sediment) (Steele, 2019).
Gastrointestinal Motility and Integrity
The GI system includes the GI tract (oesophagus, stomach, small intestines, large intestines) as well as the liver, gall-bladder, and pancreas. The GI system is responsible for the absorption and digestion of nutrients and the expulsion of waste material (Scalf, 2014). This requires effective GI motility and an intact mucosal barrier for normal digestive activity and protection of the body from GI luminal contents (Klaus, 2017).
GI tract motility conditions include megaoesophagus, gastric emptying disorders (i.e. gastritis, mechanical obstruction, ileus, ulcers, electrolyte imbalance), small intestinal transit disorders (i.e. enteritis, postsurgical ileus) (Figure 2), and megacolon (Dowling, 2015). GI tract integrity conditions include hypoperfusion (i.e. shock), gastric or intestinal foreign body ingestion, gastric ulceration (i.e. foreign body, prolonged non-steroidal anti-inflammatory drug (NSAID) usage, stress), perforation (i.e. prolonged ulceration) and traumatic penetration (Devey, 2016). Fur-thermore, when the GI tract integrity becomes compromised from critical illness, there is a risk for gut mucosal breakdown and bacterial translocation into the abdominal cavity (Campbell and Norkus, 2019). The primary clinical signs associated with GI dysfunction include hyporexia, anorexia, nausea, vomiting, and diarrhoea.

GI tract dysfunction in the critically ill can complicate disease processes and impact patient recovery (Klaus, 2017). To support the GI tract, the main pharmacological agents used to address GI symptoms include antiemetics, gastroprotectants, and motility agents (Campbell and Norkus, 2019). Centrally acting antiemetic agents (i.e. maropitant, ondansetron) work on the chemoreceptor trigger zone to block stimulation of the vomiting centre. Gastroprotectants (i.e. famotidine, omeprazole) work to block histamine receptors in the stomach to reduce gastric acid production. Motility agents, such as prokinetics (i.e. metoclopramide) work by stimulating GI peristalsis and movement (Devey, 2016).
Monitoring of GI motility and integrity involves assessment of patient parameters (i.e. temperature, heart rate, respiratory rate, blood pressure), measuring/quantifying GI losses (vomit, diarrhoea), and point of care diagnostics. Laboratory values that should be evaluated include a minimum database of a PCV, TP, blood glucose, renal values (BUN, creatinine), and electrolytes. Diagnostic imaging monitoring for the GI tract includes either radiography or an abdominal focused sonogram for trauma (aFAST) assessment (Klaus, 2017).
Nutrition
Nutrition is an important but often discounted aspect of caring and managing critically ill patients. Nutrition refers to obtaining the essential nutrients of water, protein, carbohydrates, fats, vitamins, and minerals in order to provide nourishment for overall health. Nutritional support should be provided to any patient with an illness that would limit intake of adequate nutrients, as it is necessary for recovery from all disease processes (Wortinger, 2019). The goals of providing nutritional support are to meet nutritional requirements, reverse metabolic consequences of lack of nutrition, and improve patient recovery time (Tonozzi, 2017).
When developing a nutritional therapy plan, factors such as how a patient should be fed, how much a patient should be fed, what a patient should be fed, and how often a patient is to be fed should be taken into consideration (Chan, 2016). To address how a patient should be fed, the preferred method of providing nutrition in a hospital setting is enterally. Enteral refers to nutrition that is provided via the GI tract. Providing nutrition enterally is the most physiologically appropriate method of feeding, as doing so helps maintain GI mucosa, prevents bacterial translocation from the gut, and increases wound healing and the immune response (Chan 2016; Wortinger, 2019). Since most critically ill patients are unwilling or unable to meet their nutritional requirements by mouth, a method of enteral in-tervention is via nasal feeding tubes (Figure 3). The use of naso-oesophageal and nasogastric feeding tubes are minimally invasive, well-tolerated, and relatively short-term methods of providing convenient and consistent nutritional support (Campbell and Harvey, 2012; Chan, 2012) (Figures 4a, 4b). To address what a patient should be fed, the resting energy requirement (RER) needs to be calculated. RER is the energy that is required for a normal animal (expressed in kilocalories) and is thought to be the nutritional requirement that is most similar to the nutritional needs of a hospitalised patient (Chan, 2012, 2016). The universal formula to calculate RER is (kg x 70)0.75 (Wortinger, 2019). To address what a patient should be fed, factors to consider include a patient's age, body condition score (BCS), caloric requirements (RER), and underlying disease process (Scalf, 2014). To address how often a patient is to be fed, the options are either bolus enteral feedings (liquid or blended diets delivered in larger quantities over a prescribed frequency) or trickle enteral feeding (liquid diets delivered as a continuous rate infusion) (Campbell and Harvey, 2012; Tonozzi, 2017). Regardless of the method, when instituting enteral support, it is the general recommendation to gradually begin feeding at a fraction of the patient's RER (one-quarter or one-third) to reacclimate the GI tract to nutrition and prevent the life-threatening complication of refeeding syndrome (Chan, 2016; Wortinger, 2019).
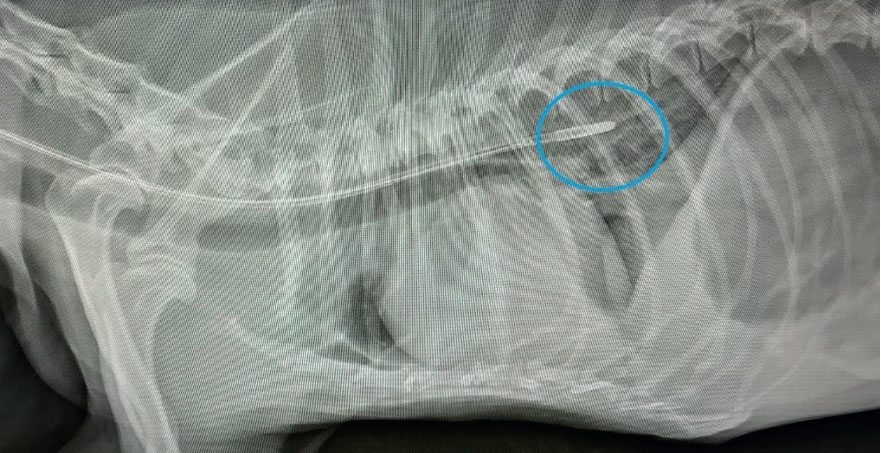
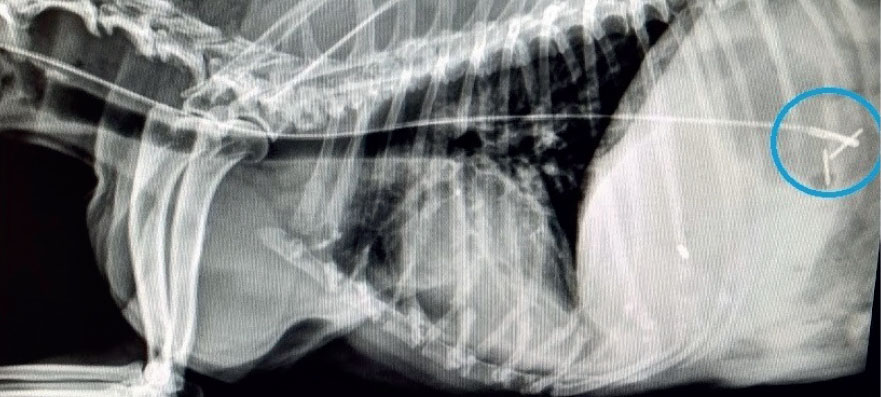
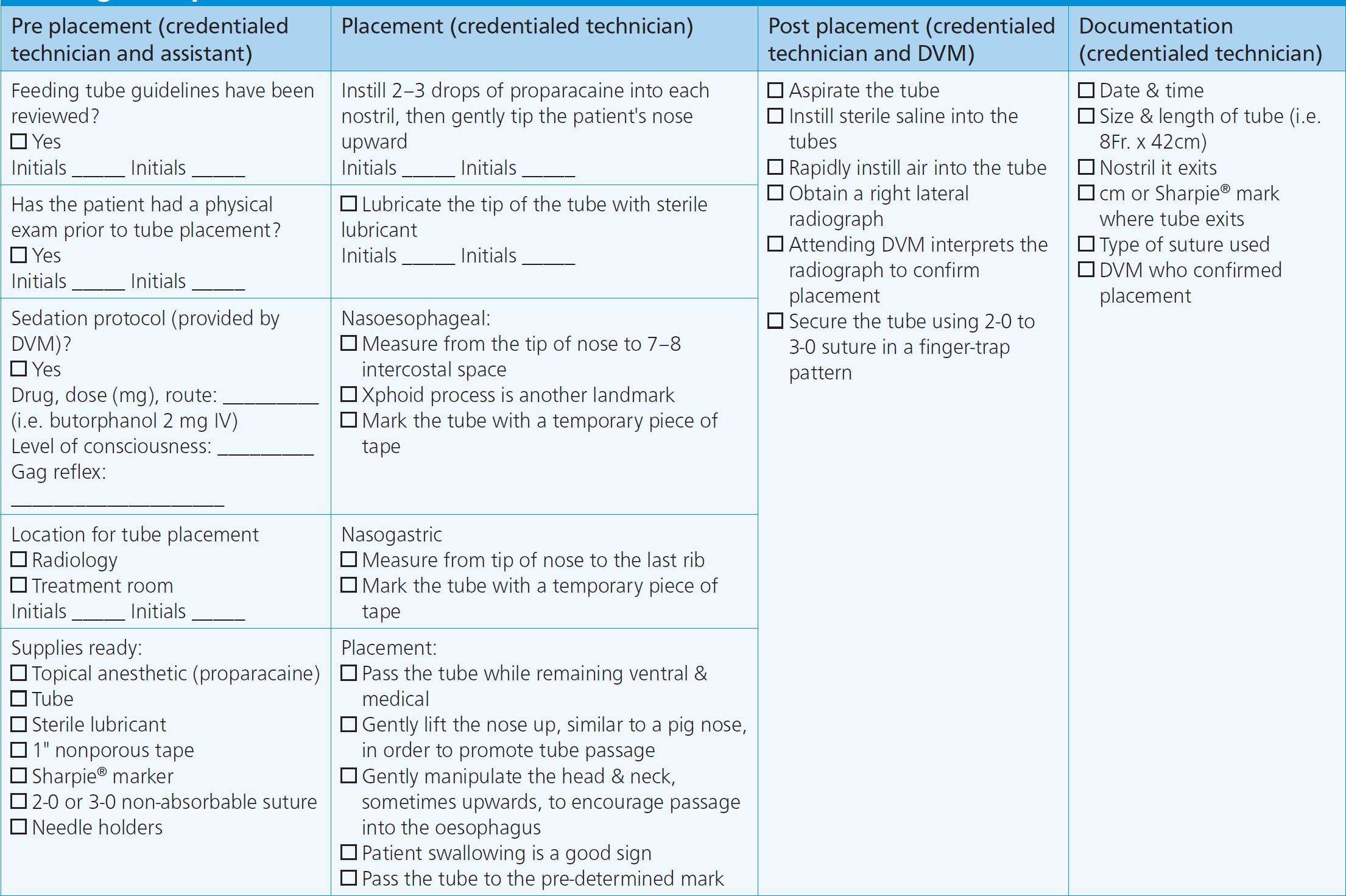
Nutrition is monitored using a nasal feeding tube checklist, assessing patient parameters, and providing nutritional support. When placing nasal feeding tubes, a checklist that can be used is shown in Figure 5. When utilising nasal feeding tubes, the tube itself should be observed for signs of occlusion, non-patency, or mal positioning that would impede nutrition administration (Campbell and Harvey, 2012). Patient parameters to assess include hypersalivation, nausea, abdominal distension, or restlessness, as any of these could be indicative of intolerance of enteral feeding. Whether enteral nutrition is being administered as a bolus feeding or trickle feeding, the amount of food should be recorded daily to ensure adequate RER is met. In addition to the amount of enteral diet administered, a patient should be monitored for signs of voluntary interest in food (if there is interest, they should be allowed to eat). Once a patient consistently consumes enough nutrients on their own to maintain RER, the calculated enteral support can be weaned (Wortinger, 2019).
Glucose
Glucose, a simple carbohydrate, is considered the body's primary energy source and is utilised by every cell (Reineke, 2012). Life-sustaining activities such as neurological function, heart rate and rhythm, vasomotor tone, respiration, regulation of metabolism, and maintaining immune status all are dependent on glucose being available to cells (Loose, 2017). In addition to energy production, glucose contributes to osmolality (water and electrolyte balance) which influences total body fluid distribution (Loose, 2017).
Euglycaemia is sustained by balancing glucose production, storage, and release, all of which can be affected from critical illness (Koenig, 2015). The normal range for blood glucose is 3.6–9.4 mmol/litre in dogs and cats, with hypoglycaemia being recognised as a blood glucose level less than 3.3 mmol/litre and hyperclycaemia being recognised as a blood glucose level greater than 9.7 mmol/litre (Reineke, 2012). Causes of hypoglycaemia include dysregulation of regulatory hormones, decreased glucose production, increased glucose consumption/utilisation, or increased glucose loss (Koenig, 2015; Loose, 2017). Examples of hypoglycaemia disorders include neurologic injury, hepatic dysfunction, endocrine disease (i.e. hypoadrenocorticism, Fanconi syndrome), toxin ingestion, sepsis, or starvation (Farry and Norkus, 2019). Causes of hyperglycaemia include dysregulation of regulatory hormones, reduced glomerular (renal) filtration, or stress (Koenig, 2015; Loose, 2017). Examples of hyperglycaemia disorders include neurologic injury, endocrine disease (i.e. diabetes mellitus), or renal dysfunction (Farry and Norkus, 2019).
Blood glucose is monitored using general patient observation and point of care testing. A patient should be assessed for clinical signs associated with either hypoglycaemia (i.e. altered level of consciousness, weakness or ataxia, hypotension, acute collapse, muscle tremors or twitching, seizure activity) and hyperglycaemia (altered level of consciousness, weakness, dehydration, polyuria, polydipsia, polyphagia) (Scalf, 2014; Loose, 2017). Point of care testing involves instant measurement of blood glucose levels via a glucometer. Additional laboratory values may need to be evaluated based on the underlying disorder causing the alteration of blood glucose.
Immune Status and Antibiotics
The immune system is the body's complex defense mechanisms against invasion of microorganisms (i.e. bacteria, viruses, fungi, protozoa, parasites) that cause disease (Scalf, 2014). The primary function of the immune system is to protect the body from anything that could cause harm, damage or disease. An antigen is any foreign substance that when introduced to the body, stimulates an immune response; when the body detects an antigen that threatens its health, it employs various mechanisms to destroy it (i.e. leukocyte recruitment, antibodies) (Haak, 2017). An antibody (also known as an immunoglobulin) is a Y-shaped protein used by the immune system in response to an antigen; its role is to neutralise the antigen by binding to it and making the antigen inactive (Haak, 2017). Dysfunction of the immune system can result in a patient being unable to mount a rapid, aggressive, and effective immune response to protect itself from invasion.
The immune system of the critical patient can be affected by patient factors, disease factors, and environmental factors, including immunosuppression (i.e. leukopenia), GI dysfunction (i.e. risk for bacterial translocation, aspiration, malnutrition), autoimmune disease processes (i.e. immune-mediated haemolytic anaemia, immune-mediated thrombocytopenia), wound contamination, infectious disease (i.e. pneumonia, sepsis, pancreatitis, parvoviral enteritis, pyelonephritis, leptospirosis, pyometra), and nosocomial infections (Bays and Foltz, 2019).
The immune system is monitored through patient parameters, assessment of laboratory values, and patient handling. Patient parameters that should be assessed include the six perfusion parameters (mentation, heart rate, pulse rate, mucous membrane colour, capillary refill time, extremity temperature), body temperature for pyrexia, hydration status, and bodyweight. The primary laboratory value to assess is white blood cells (WBC) through either the leukogram portion of a complete blood count (CBC) (total WBC, complete differential, WBC morphology) or a manual WBC differential via blood smear (Haak, 2017). When handling patients with an immune disorder/response for assessment and monitoring, care should be taken to follow thorough hand hygiene and donning of examination gloves during each patient interaction and changing gloves between patients.
Antibiotic drugs (antimicrobials) are among the most common medications prescribed for critically ill patients and are indicated when there is evidence of infection (i.e. immune dysfunction). The goal of antimicrobial therapy is to safely eliminate infection by practicing antimicrobial stewardship to minimise the emergence of antimicrobial resistance (Boothe, 2015; Haak, 2017). Effective and applicable antimicrobial stewardship includes identification of the infectious pathogen, determining pathogen susceptibility (i.e. culture results), timely selection of the most appropriate drug, drug dose, and dosing interval for the pathogen, and de-escalation if alternative therapies are equally effective or when the infection is controlled (Boothe, 2015; Haak, 2017).
Antibiotic usage in the hospital setting is monitored through creating and following an antibiotic protocol and having a structured antimicrobial stewardship programme. This involves taking into consideration an antibiotic's action and spectrum, utilising combination therapy, charting of culture susceptibility, antibiotic rotation, hospital hygiene and barrier/isolation nursing techniques if/when indicated (Haak, 2017; Bays and Foltz, 2019).
Conclusion
Veterinary nurses are the patient's primary caregivers and are involved in monitoring critical patients. Utilising the Kirby's Rule of 20 patient checklist provides an organised method for veterinary nurses to thoroughly assess the critical patient parameters of renal function, GI motility and integrity, nutrition, glucose, and immune status and antibiotics. Having a thorough understanding of each of these patient parameters enables veterinary nurses to prioritise patient needs by assessing the overall clinical picture to elevate the standards for quality patient care.
Key Points
- Kirby's Rule of 20 is an established, evidence-based patient checklist that can be used by veterinary nurses in the emergency and critical care setting to assess the overall clinical picture of critically ill patients, implement critical thinking skills, and elevate the quality of patient care.
- Renal function involves the roles of maintaining fluid balance, removing metabolic waste from the body, regulating blood pressure, and producing urine. Acute kidney injury is a secondary condition that can be caused from various disease states, which requires monitoring of renal parameters.
- Gastrointestinal (GI) motility and integrity refers to the structures that maintain effective GI motility and an intact mucosal barrier to have normal digestive processes. GI dysfunction can occur from primary or secondary disease processes that require monitoring and supportive care.
- Nutrients are essential to life and therefore nutritional support is an important aspect of critical patient care. Providing nutrition involves developing a nutritional therapy plan that takes into consideration a patient's disease process, dietary needs, method of feeding, and monitoring of patient tolerance.
- Glucose is considered the body's primary energy source and is utilised by every cell to maintain homeostasis. Several disease states can affect a patient's glycaemic index and it therefore requires monitoring using point-of-care testing.
- Immune status refers to the body's defense system to protect it from disease. Antibiotic stewardship involves safely eliminating infectious sources while minimising antimicrobial resistance. Critically ill patients should be monitored for signs of infection and use of antibiotics should follow a structured hospital protocol.


