For centuries there has been evidence of the use of light to promote healing (Hamblin et al, 2018). In 1903, The Nobel Prize in Medicine and Physiology, was awarded for the use of phototherapy in the successful treatment of disease (Tata and Waynant, 2011). Dr Endre Mester is considered the founding father of laser biostimulation; he used low power red light to treat humans and animals, demonstrating reduced wound healing times (Tata and Waynant, 2011; Paterniani and Grolli, 2017).
Throughout the evolution of laser therapy devices multiple names have emerged that describe both the technology and device itself; these include low-level laser therapy, low-level light therapy, LLLT, therapeutic lasers, cold laser, soft laser, photobiostimulation and phototherapy, all of which are different names for the same thing. In 2016, photobiomodulation therapy (PBMT) was agreed as the preferred and standardised term to be used going forward (Anders et al, 2015).
Definition of photobiomodulation therapy
PBMT is a form of light therapy that uses non-ionizing light sources, such as lasers, light emitting diodes (LED) light and broadband light within both the visible and infrared spectrums. PBMT is a non-thermal process that stimulates endogenous chromophores with subsequent biological effects. This results in beneficial therapeutic outcomes including pain relief, the reduction of inflammation, immunomodulation and the promotion of wound healing and tissue regeneration (Anders et al, 2015).
What it is/how it works?
Light amplification by stimulated emission of radiation (laser) is a monochromatic, coherent and collimated beam, while LED is an incoherent beam (Riegel and Sepion, 2007). The appropriate application within the ‘therapeutic window’ allows laser and LED wavelengths to cause a biostimulatory effect in target tissues (Hamblin et al, 2018). Each cellular target absorbs specific wavelengths of light that initiate cellular processes resulting in biologic effects. These cellular processes include adenosine triphosphate (ATP) synthesis, enzymatic activity, and DNA production (Kraut et al, 2013). The most well described mechanism focuses on the terminal enzyme of the mitochondrial electron transport chain, chromophore cytochrome C oxidase. Inhibitory nitric oxide is dissociated by photons from the cytochrome C oxidase, increasing electron transport, ATP production and mitochondrial membrane potential (Looney et al, 2018).
The main therapeutic effects of PBMT are anti-inflammatory and anti-oedema effects, analgesic effects and the regeneration of damaged tissues (Paterniani and Grolli, 2017). The clinical results of PBMT rely on the irradiation parameters (wavelength, irradiance and coherence) and light dose parameters (energy, energy density, irradiation time and treatment intervals) administered (Table 1).
Table 1. Irradiation and light dose parameters of photobiomodulation therapy
| Irradiation parameter | Measurement unit |
|---|---|
| Wavelength | nm |
| Irradiance | Watts/cm2 |
| Coherence | Spectral unity, logic and width |
| Energy | J (Joules) |
| Energy density | J/cm2 |
| Irradiation time | Seconds |
| Treatment Interval | Days/weeks |
Wavelengths of red or near-infrared light (650–904 nm) have biostimulatory effects. Lower energy densities (1–4 J/cm2) are used for the treatment of superficial tissues and higher energy densities (8–12 J/cm2) are used for deeper tissues (Paterniani and Grolli, 2017). A biphasic response to PBMT is observed, with gradual dose increases until the benefits reach a maximum point. Further dose increases above this threshold result in a decline in response (Hamblin et al, 2019).
Use of PMBT
The application of PBMT in veterinary medicine is common in domestic species, but use is increasing for exotic and zoo species as well (Paterniani and Grolli, 2017). PBMT is a complementary treatment option that is non-invasive, painless and is easy to apply (Venter, 2013). In zoological collections, using positive reinforcement training to elicit husbandry and medical behaviours, PBMT is easy to administer with minimal intervention and stress for the animal. Positive reinforcement training allows repeat treatments to be performed without the need for anaesthesia or restraint.
The pre-programmed settings on machines allow for a wide variety of applications in veterinary practice (Venter, 2013). However, because of the variability in zoo patient size, skin thickness, coloration, and depth of target tissues, it is challenging to determine an appropriate energy dose for target tissues; species specific treatment protocols are required (Dadone and Harrison, 2019). Even with the use of positive reinforcement training, the protective contact distance required to work safely with many zoo species can result in significant distance between the target tissue and the probe, likely reducing efficacy of the treatment.
Additionally, because many cases are complex, patients often receive concurrent therapies. The medical benefits of PBMT alone are generally anecdotal or difficult to prove and interpret (Dadone and Harrison 2019). The Zoological Society of London (ZSL) London Zoo uses a class 3b Omega Laser Systems XP mobile unit (Omega Laser Systems, Halstead, CO9 2RT, UK) with four probes: 820 nm 200 mW; 5 x 820 nm 200 mW; 660 nm 50 mW; and 20 Diode Cluster. PBMT is used alongside medical management and is incorporated into cases at ZSL, most commonly for wound healing, pain relief and arthritis management.
An individual PBMT plan is created for each animal that details the wavelength, energy density, treatment time and frequency that will be administered. Animal training plans may also be developed, prior to treatment, to facilitate safe and reliable access to the patient and the treatment area. The PBMT plan requires regular review and is updated based on the clinical effects of treatment.
Wound healing
In wound healing, PBMT modulates both systemic and local responses (Hussein et al, 2011). The stimulation and acceleration of cell reproduction and growth leads to faster repair of damaged tissues and the moderation of the inflammatory response (Bradley, 2017). PBMT also improves collagen deposition and density in wound healing, as well as a reduction in oedema (Rychel et al, 2011).
The promotion of surgical wound healing in rabbits treated with PBMT was demonstrated by Hussein et al (2011). There was histological evidence of faster resolution of the acute inflammation, allowing the proliferation phase of wound healing to happen earlier compared with controls, indicating that PBMT decreased the inflammatory reaction of wound healing. This was replicated in rats, showing that a single treatment stimulated wound healing by reducing the inflammatory reaction. However, after 14 days no significant differences in healing were observed between the treatment and non-treatment groups (Sabater Gonzalez and Mayer, 2019).
Wound healing in reptiles progresses using the same stages as mammalian healing albeit at a slower pace (Mader et al, 2006). Despite the limited literature available, there is some evidence that PBMT has been used to stimulate and accelerate tissue repair in reptiles (Paterniani and Grolli, 2017). In one study, wounds in iguanas treated with 10 J/cm2 were significantly smaller when compared with those treated at 5 J/cm2 or those that received no treatment (Mayer and Ness, 2017). Kraut et al (2013) used high power (2–2.5 Watts) with a defocusing handpiece, held 10 cm away from the treatment area to treat shell ulcerations in a soft-shelled turtle. A review of the wounds demonstrated that irradiated sites healed better and faster than those left untreated (a decrease in depth and change of colour), despite no difference in size (when measuring outside wound margins) of the lesions. This result suggests benefits associated with the use of PBMT, but because treatment was multimodal and husbandry issues were also addressed, this is hard to prove.
In all species daily treatment for 3 consecutive days, then decreasing to every other day, is recommended for surgical or acute wounds, with a dose of 1–4 J/cm2. Chronic wounds need a much higher dose rate of 4–30 J/cm2, and often require a prolonged and aggressive treatment regimen, particularly if lesions are granulomatous or infected. Daily PBMT is recommended initially for 3–5 treatments then reduced to twice weekly thereafter, until the condition has healed, resolved, or plateaued (Bradley, 2017).
ZSL wound healing cases
Aardvark (Orycteropus afer)
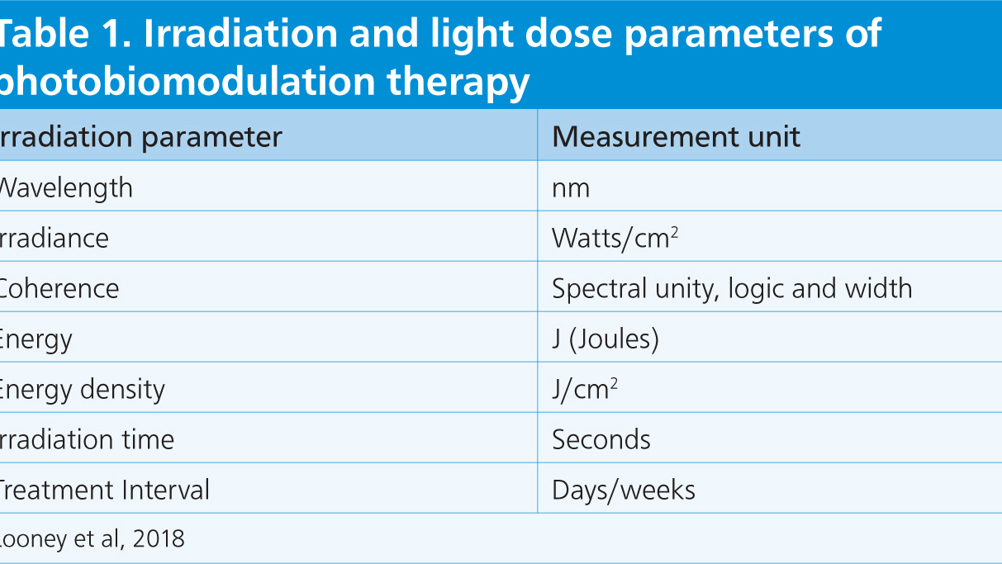
PBMT was first used at ZSL as a component of a wound treatment plan for a 3-year-old male aardvark. The animal arrived at the zoological institution with wounds over its prominent lumbar vertebrae, thought to have been caused by trauma while in transit from another collection. Despite treatment (meloxicam 0.15 mg/kg every 24 hours orally (Inflacam®, Virbac); amoxicillin clavulanic acid 12 mg/kg every 12 hours orally (Clavaseptin®, Vetoquinol); and daily topical aluminium spray (Aluspray®, Vetoquinol)), the wounds appeared static having showed no signs of improvement. Intensive treatment was initiated; the wounds were cleaned with chlorhexidine gluconate (HiBiScrub®, Möln-lycke Health Care Ltd) and flushed with saline (Aqupharm 1 ® (9 mg/ml) solution for injection/infusion, Animalcare) every other day. Dressings were applied as a barrier to prevent gross wound contamination from enclosure substrate, and non-steroidal anti-inflammatory drugs (NSAIDs) and antibiosis were administered systemically, based on culture and sensitivity results.
PBMT was initiated with 3 consecutive days of treatment using 660 nm, 8 J/cm2, 20 Hz at the wound margins, and 660 nm, 8 J/cm2, 700 Hz and LED 6 J/cm2 with multi-pulse frequency 146-73–2.5 Hz over the wound surface. The sessions were then continued 2–3 times a week to coincide with dressing changes and wound assessment. After the first 5 sessions, the energy density was alternated between 6 J/cm2 and 8 J/cm2. A total of 15 PBMT sessions were carried out over a 6-week period resulting in wound closure (Figure 1). Aardvarks are nocturnal so treatment was completed during daytime sleeping periods with minimal disturbance to the animal.
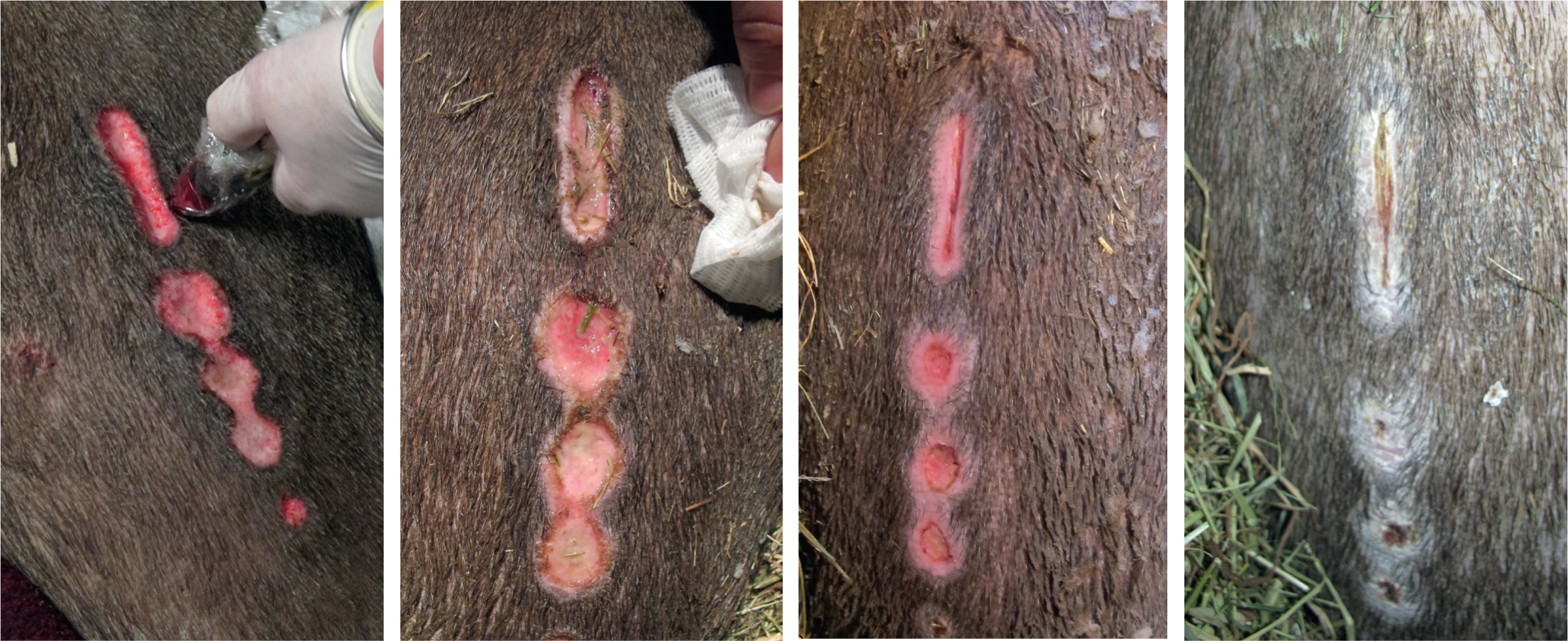
Big-headed turtle (Platysternon megacephalum)
A 7-year-old female big-headed turtle presented with ulcerative carapacial lesions, two on the pleural scutes and one on the marginal scutes (Figure 2). No further abnormalities were found on clinical examination; haematology and biochemistry were within normal limits. The lesions were debrided and cleaned with chlorhexidine gluconate (HiBiScrub®, Mölnlycke Health Care Ltd), flushed with saline (Aqupharm 1 ® (9 mg/ml) solution for injection/infusion, Animalcare), and silver sulfadiazine cream (Flamazine, Smith & Nephew) was applied topically every other day for a week. Concurrent antibiotics and anti-inflammatories were administered and changes to husbandry included the introduction of salt to the water at 0.5 g/litre.
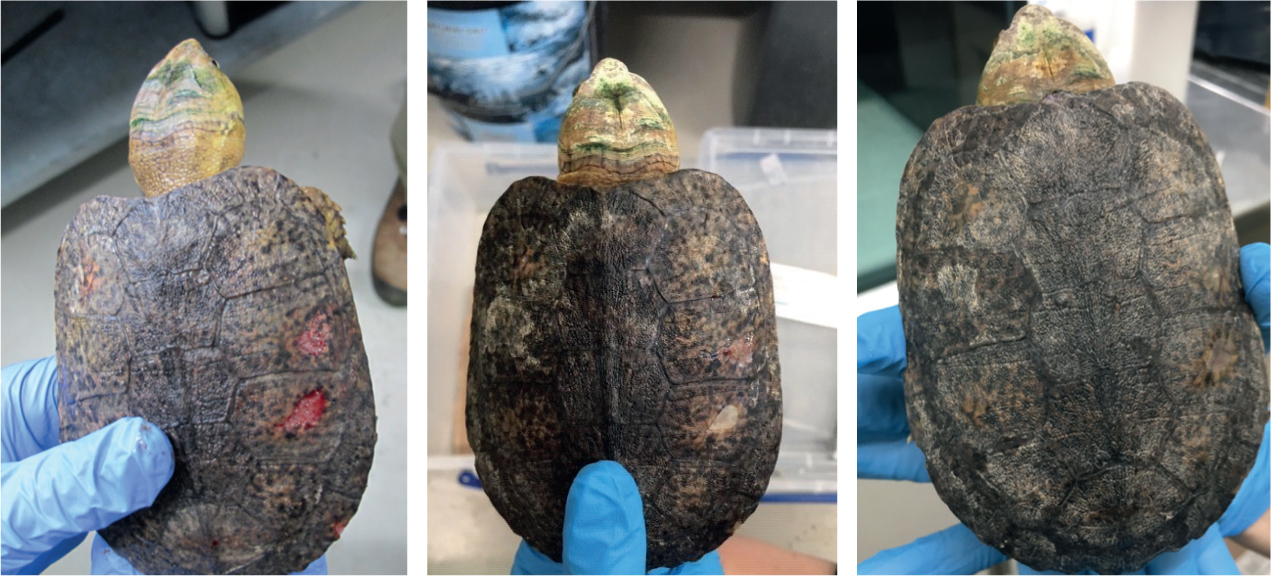
PBMT was initiated using the LED probe, with a 1 minute cycle at 6 J/cm2 and a multi-pulse frequency of 2.5 Hz, 20 Hz and 73 Hz. The probe was held perpendicular to the lesions and 1–2 mm from the lesion surface. PBMT treatment occurred after cleaning, but before the application of topical treatments.
The frequency of the PBMT was reduced throughout the treatment period to coincide with concurrent handling and therapy administration. After the second week, treatment sessions were reduced to twice weekly as the application of topical treatment had been discontinued and the lesions were reducing in size, with the presence of normal tissue repair and regrowth noted. A total of eight PBMT sessions were carried out over 42 days.
Musculoskeletal conditions
PBMT can have almost immediate analgesic effects as observed in the management of a green moray eel (Gymnothrax funebris) with vertebral misalignment, compression of vertebral centra and ventral osteocyte formation. After the first PBMT session (6161 J, 8 W for 2–3 minutes) the eel swam away from the handler with normal motion that had not been previously observed (Boylan et al, 2016). A chronically lame reticulated giraffe (Giraffa camelopardalis reticulata) treated with PBMT voluntarily participated in hoof trims within 48 hours of treatment, a behaviour not exhibited for months previously. Thermography also showed a reduction of surface temperature in the affected right hind limb (Dadone and Harrison, 2017).
The combinations of medical management, PBMT, range of motion (ROM) exercises and chiropractic adjustments to manage acute-onset torticollis in a reticulated giraffe (Giraffa camelopardalis reticulata) resulted in a marked clinical improvement (Dadone et al, 2013).
Benefits are also observed when PBMT is used for the management of chronic conditions. Using PBMT for its analgesic and anti-inflammatory effects may reduce or eliminate the need for medications (Johnson, 2017). Looney et al (2018) demonstrated a reduction in the original dose of NSAID required by 50% of patients with canine elbow osteoarthritis receiving adjunctive PBMT treatment. There was also a decrease in the lameness score and an improvement in the pain score of individuals treated with PBMT. The treatment regimen was 10–20 J/cm2, 5–10 W per joint, twice a week for 3 weeks, then once weekly for 3 weeks.
In an experimental osteoarthritis trial in rats, one treatment group received PBMT and one received PBMT and concurrent topical NSAIDs. PBMT alone was demonstrated to effectively modulate the underlying inflammatory process (6 J per joint 100 mW over 60 seconds) with better outcomes than the combined treatment group (Tomazoni et al, 2017).
Long-term PBMT plans are required for chronic cases; plans including chronic osteoarthritis pain also require continued maintenance therapy. Initial treatments should be frequent (three or four times a week), then tapering slowly to a maintenance therapy, which can be as little as every 4–6 weeks (Johnson, 2017).
ZSL musculoskeletal conditions
Ring-tailed lemur (Lemur catta)
PBMT is recommended as a management tool for chronic pain in geriatric mammals (Dadone and Harrison, 2017). PBMT was incorporated into a management plan for a geriatric (26-year-old) male ring-tailed lemur with thoracolumbar spondylosis and bilateral arthritic stifles with new bone growth and reduced flexion. The individual was already receiving an oral NSAID (firocoxib 6.5 mg/kg; Previcox®, Boehringer Ingelheim Animal Health UK Ltd) once a day and oral gabapentin (5.0 mg/kg; Gabapentin oral solution, Rosemont® Pharmaceuticals Ltd) twice a day. He also received a glucosamine and green-lipped mussel (150 mg glucosamine/100 mg ActiveEase® green-lipped mussel (Perna canaliculus)/0.75 mg hyaluronic acid; YuMOVE Cat®, Lintbells) joint supplement daily. PBMT was initiated with a frequency of three times weekly, then reduced to once weekly over a period of 6 weeks. Treatment was carried out when the animal was stationed to a log within the enclosure to receive medication and breakfast foods. The length of the treatment sessions varied from 4–9 minutes depending on the animal's willingness to participate. A 5 x 820 nm probe was used to cover a larger surface area at 24 J/cm2 with a pulsing frequency of 5 kHz. Treatment areas included the stifles bilaterally.
Although significant changes were not observed after the introduction of PBMT, it is the author's belief, based on the literature regarding PBMT mechanism and therapeutic effects, that treatment was beneficial. The lemur maintained his position as the dominant male within the bachelor group. Enclosure usage and gait assessment showed some compensation with alternative pathways sought however, the lemur was able to navigate the enclosure to access all resources easily. Finally, the gabapentin dose was at the low end of the dose range for chronic pain management extrapolating from data and dose ranges for cats (5–10 mg/kg orally, every 8–12 hours) (Ramsey, 2017).
Military macaw (Ara militaris)
A 2-year-old female military macaw received PBMT as part of a multi-treatment approach to flexor tendon mineralisation. Intermittent lameness and associated soft tissue swelling, along with reduced ROM was evident. Oral analgesic treatment included meloxicam (1 mg/kg; Inflacam ®, Virbac) once daily and tramadol (15 mg/kg; Tramadol, Goldshield) twice daily.
PMBT sessions were initiated after diagnosis, beginning with three times weekly for 1 month, reduced to twice weekly, then once weekly. There was evidence of deterioration and a reluctance to bear weight on the affected limb when PBMT sessions were reduced to once a week. Therefore, PBMT was increased and maintained twice weekly thereafter. Treatment times varied from 2–5 minutes depending on the bird's willingness to participate in training sessions. The 5 x 820 200 mW probe was used to treat a large surface area in a reduced amount of time. The energy density was 8 J/cm2 with pulsing frequency alternating between 1 kHz and 5 kHz each session. A lower power could have been considered, but higher levels were chosen because of the distance between the treatment area and the probe.
With regular twice weekly PBMT and analgesia, the macaw appeared to be using the limb comfortably. The deterioration seen after reducing PBMT frequency indicates that there were some anti-inflammatory and analgesic effects with PBMT. There were no changes made to the oral medication during this time, however, external factors such as a traumatic event cannot be ruled out to account for the deterioration.
Conclusions
There is significant evidence that PBMT is a useful adjunctive therapy option and is generally easily applied because of its non-invasive nature. PBMT is increasingly being incorporated into the management of cases at ZSL. Outcomes and efficacy are evaluated on a case-by-case basis, however, there is no straightforward way to standardise this assessment across species and conditions. Multimodal treatment approaches, training participation reliability and access to treatment areas are further complicating factors in some cases.
Based on the biological effects described in the literature, there is an ethical argument advocating for the incorporation of PBMT into treatment regimens where benefits could occur. However, treatment plans must be tailored to the individual animal while considering species variability and the medical condition(s) being treated. The regular assessment of therapeutic effects is essential in determining the best clinical results. The continued use of PBMT in zoo species will help further develop the understanding of the most effective irradiation and light dose parameters in a variety of species.
KEY POINTS
- Photobiomodulation therapy (PBMT) is a form of light therapy that uses non-ionizing light sources, such as lasers, light emitting diode (LED) light and broadband light within both the visible and infrared spectrums.
- Beneficial therapeutic outcomes from PBMT include pain relief, the reduction of inflammation, immunomodulation and the promotion of wound healing and tissue regeneration.
- PBMT is a complementary treatment option that is non-invasive, painless and is easy to apply.
- Because of the variability in zoo patient size, skin thickness, coloration, and depth of target tissues, it is challenging to determine an appropriate energy dose for target tissues therefore species-specific treatment protocols are required.
- Patients often receive concurrent therapies, hence the medical benefits of PBMT alone are generally anecdotal or difficult to prove and interpret.