Osteoarthritis (OA) is seen commonly in dogs with a suggested 20% of the population suffering from the condition (Marshall et al, 2009). There is no cure for OA and patients will commonly be suffering from discomfort, limited range of motion, muscle atrophy and reduced mobility (Bockstahler et al, 2004). Current management strategies include the use of analgesics, nutraceuticals, physiotherapy, and alternative therapies such as acupuncture. Hydrotherapy is a branch of physiotherapy which utilises the properties of water, such as buoyancy, resistance, and hydrostatic pressure, to aid in the rehabilitation of patients and is commonly advocated in the long-term management of OA (Millis and Levine, 1997). Currently, little research is available for the use of hydrotherapy in the treatment of canine OA; however, despite this lack of evidence a survey conducted by Waining et al (2011) found it to be the third most common condition seen in UK hydrotherapy centres. This article will discuss the aetiology and pathogenesis of OA, why hydrotherapy could be beneficial, and the current evidence that is available to support its use.
Osteoarthritis
The initial cause of OA can be difficult to determine as there may be a number of factors contributing to the development and progression of the condition. Johnston (1997) summarises the causes of osteoarthritis into two categories: normal forces on abnormal cartilage, or abnormal forces on normal cartilage. Abnormal forces can be generated by incongruence or instability in the joint and can be due to developmental abnormalities such as hip dysplasia and elbow dysplasia, or injuries such as joint fractures or ligament damage (Johnston, 1997; McLaughlin, 2000). Additionally, obesity may result in excessive forces being applied to joints (Raditic and Bartges, 2014) and research has shown a link to the development of hip OA in Labradors (Smith et al, 2006). Abnormality of the cartilage tissue can be primarily caused by conditions such as osteochondrosis dissecans (Robins and Innes, 2006). Additionally, it has been shown in humans that the ageing process leads to changes in the cartilage tissue which make it more vulnerable to damage (Martin and Buckwalter, 2002). It was previously considered that cartilage degenerated with age (Fossum et al, 1997) and the development of OA was inevitable. However, Martin and Buckwalter (2002) refute this suggesting it is not an inevitable result of ageing but that age-related changes weaken the cartilage and increase the risk of damage. There is also much discussion over the role of exercise in the development of OA. A review of human research by Bosomworth (2009) established that moderate exercise (in the absence of injury) is not a risk factor in the development of OA, however, more strenuous exercise may be a factor in the development of OA. In any case irrespective of the primary cause once initiated the progression of osteoarthritic changes is similar in all cases and is often self perpetuating.
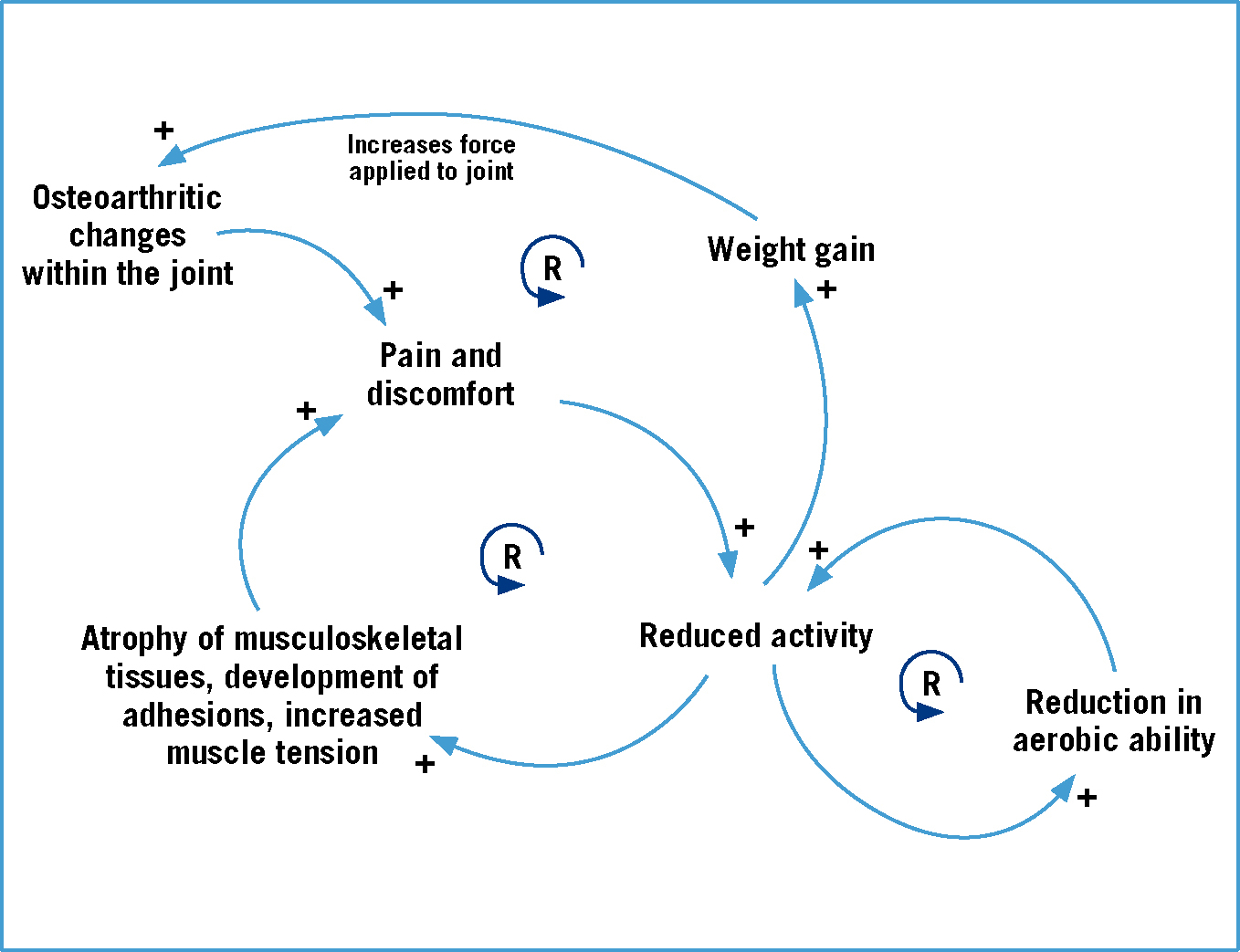
The pathogenesis of OA is a complex process that involves a combination of biochemical, physical and pathological changes (Johnston et al, 2008). It is often described as the degeneration of articular cartilage (Abercomby et al, 2006), but changes are also recognised in other structures that are integral to the normal functioning of the joint such as the synovial membrane, synovial fluid, joint capsule and subchondral bone. Johnston (1997) suggests that microscopic roughening of the uppermost layer of the cartilage is the first change seen in OA. The subsequent loss of cartilage fragments into the synovial space initiates an inflammatory reaction within the joint and the release of degradative enzymes, which break down the cartilage further, along with inflammatory mediators which serve to exacerbate the response (McLaughlin, 2000). Damage to the integral structure of the upper layers of the cartilage also results in the exposure of lower layers and the subchondral bone to abnormal stresses. This may result in damage occurring or, in the case of the subchondral bone, thickening which make it less deformable on loading (Johnston, 1997). The extracellular matrix of the cartilage is made up of a mesh work of collagen, proteoglycans and water and it is the particular balance and arrangement of these molecules that allows cartilage to withstand tensile and compressive forces (Mclaughlin, 2000). It should therefore be appreciated that even a small amount of upset within this arrangement can have drastic effects on its ability to withstand such forces and so further damage results, illustrating the self-perpetuating nature of this disease. Other changes seen within the osteoarthritic joint include the synovial fluid becoming less viscous and an increased stiffness in the joint capsule. The latter may be related to the subsequent reduced activity of patients; Millis and Levine (1997) discuss how as OA progresses a patient's activity level will decline due to associated pain and discomfort. This reduced activity leads to changes in the musculoskeletal tissues (Table 1), reduced fitness levels, and may lead to weight gain, and so a vicious cycle commences (Figure 1).
Table 1. The effects of disuse on musculoskeletal tissues
| Tissue type | Changes associated with disuse |
|---|---|
| Cartilage |
|
| Joint capsule |
|
| Muscle |
|
| Ligaments & tendons |
|
| Bone |
|
Properties of water and implications for therapy
The benefits of using water-based therapy in the management of OA centre on the properties of water: most importantly buoyancy, hydrostatic pressure and viscosity.
Buoyancy
When an object is immersed in water, the body of water exerts an upwards thrust on that object known as buoyancy (Prankel, 2008). This upward thrust leads to a reduction in weight transferred through the body and has been shown by a measured reduction in vertical ground reactive forces (vGRF) (Levine et al, 2010). Additionally, increasing levels of immersion provide increasing reduction in vGRF; Levine et al (2010) found that vGRF were decreased by 9% with water at the level of the tarsus, by 15% with water at the level of the stifle and by 62% with water at the level of the hips. It is important to note that, due to relative density, a patient's body composition (amount of fat tissue) is likely to have an effect on the reduction of vGRF; patients carrying more fat are more likely to float and therefore experience a greater reduction in vGRF. Buoyancy is an important property of water to aid in the management of conditions such as OA as it allows exercise to be conducted in a reduced weight bearing environment. This helps to maintain muscle mass, exercise tolerance, and range of motion, while reducing the impact on painful joints. Additionally, maintaining and improving exercise levels is important to prevent and manage weight gain.
Hydrostatic pressure
Hydrostatic pressure is the pressure exerted on an object by a body of fluid when the object is immersed in such fluid. The pressure exerted on an object is directly proportional to the depth of immersion; the deeper the object the greater the pressure (Bockstahler et al, 2004; Monk, 2007). It is believed that it is this property of water exerting pressure on the lymphatic system, improving lymphatic return, that leads to a reduction in oedema in cases where hydrotherapy has been utilised (Becker, 1997). This suggests that simple immersion in water with or without exercise could have great benefits on oedema resolution and associated pain and stiffness, although as Lindley and Smith (2010) discuss the muscle contraction associated with exercising is also likely to play a role in the reduction of oedema. Hydrostatic pressure is also suggested to help reduce pain perception during exercise due to the stimulus of the sensory receptors on the skin leading to a reduction in nociceptor hypersensitivity (Levine et al, 2014).
Viscosity and drag
Viscosity is a fluid's resistance to movement (Bockstahler, 2004) caused by the cohesion and adhesion of molecules. A patient therefore needs to exert a certain amount of force to a body of water in order to move through it, thus creating more of a challenge for the musculoskeletal and cardiovascular systems than the same exercise performed on land (Levine et al, 2014). Cureton (1997) explains though how the energy required is dependent on the activity performed, the water depth and the speed at which the activity is performed. This is due to the added buoyancy provided by the water reducing the energy required for a patient to move their own body weight offsetting the added resistance the water provides. Therefore at slow speeds exercising fully immersed in water may be less challenging than exercising on land, which could be ideal in the initial stages of OA treatment. However, with increasing speed comes increased resistance, which in turn increases the challenge on musculoskeletal and cardiovascular systems, allowing treatment protocols to be progressed.
Drag produced during water movement results in movement being 799 times slower than such a movement performed in air (Monk, 2007). For this reason weak, unstable animals, who are unable to stand on land for long periods, may be able to stand better in water as falling takes longer allowing them time to readjust their balance. This may mean osteoarthritic patients unable to stand for extended periods of time, or unwilling to exercise through fear of falling, may be more comfortable exercising in the aquatic environment.
Hydrotherapy modalities
Hydrotherapy can be applied in a number of forms: pool (Figure 2), underwater treadmill (UWTM) (Figure 3) or whirlpool/hot tub. The general principles of aquatic therapy apply in all cases, but there are differences that should be considered when deciding which is the most suitable modality to be used for an individual. Benefits of the UWTM include the ability to adjust the height of the water, a feature not possible in the pool, allowing the amount of weight bearing to be controlled. Additionally, the speed of the treadmill can be set allowing the therapist to have some control over the intensity of the exercise performed (Doyle and Liska, 2008; Chiquoine et al, 2013). It was demonstrated in the study conducted by Marsolais et al (2003) that angular velocities of the hip, stifle and hock were significantly greater during swimming than walking and this may be unfavourable during early stages of treatment. Marsolais et al (2003) also found that, when compared with walking, swimming resulted in significantly greater range of motion (ROM) of the hip, stifle and hock. However, this increased ROM was attributable to the increased flexion of the joint, with extension of the stifle and hock found to be significantly less during swimming than walking. Therefore, consideration should be made as to whether an increase in extension or flexion is preferable. Another factor to consider when deciding whether the pool or UWTM is most suitable is the patient being treated; some dogs have a fear of water and are reluctant to be lowered into the pool. In such cases the UWTM, where the water rises from the bottom and where the level can be controlled, may be better tolerated (Levine et al, 2014; Chiquoine et al, 2013). Additionally, due to the effects of hydrostatic pressure, patients with cardiorespiratory disease may be better suited to the UWTM (Lindley and Smith, 2010).


Whirlpools or hot tubs, where the temperature is higher than normal hydrotherapy pools and there may be jets that provide turbulence, are less commonly seen but can be used for static treatment. The warmth and massaging effect of the turbulence helps to increase circulation and reduce stiffness of the musculoskeletal tissues; a common source of pain in osteoarthritic patients (Johnston et al, 2008). It should be noted that the use of warm water therapy is contraindicated during acute inflammation due to the capacity to increase oedema formation.
Evidence for the use of hydrotherapy for OA
Hydrotherapy has been used in the treatment of OA in humans since the 1900s (Becker, 2009). Research has been conducted in the human field that has shown the benefit of introducing aquatic therapy in the management of OA. In 2007 a randomised, controlled trial (RCT) was conducted by Wang et al that investigated the use of an aquatic exercise programme for human patients with hip or knee OA. A sample of 42 adults was divided into two groups; one group was treated with an aquatic exercise programme and the other group continued with their usual level of activity. The results indicated a significant (p<0.05) improvement in knee and hip ROM, muscle strength and aerobic fitness (taken as a measurement of how far the person could walk in 6 minutes; 6 minute walk test) in the aquatic exercise group. However, no significant difference was seen in self-reported pain and physical function. A further RCT conducted by Hinman et al (2007), which compared aquatic therapy to no treatment in 71 human patients with hip or knee OA, reported hip muscle strength and 6 minute walk test was significantly (p<0.05) better in the aquatic therapy group. Additionally, in this trial a significant (p<0.01) improvement was seen in pain on movement, pain overall and physical function as reported by the subjects of the aquatic group.
Further RCTs have taken place in the human field to compare aquatic therapy with land-based therapy (Wyatt et al, 2001; Foley et al, 2003; Lund et al, 2008; Silva et al, 2008). As with previous studies discussed, improvements were seen over time in patients treated with aquatic exercise, however, similar improvements were seen in the land-based exercise groups with no significant difference found between groups. This potentially suggests that aquatic-based exercise is no more beneficial than land-based exercise. A comment made by Tovin et al (1994) regarding research comparing aquatic and land-based exercise may be of relevance though; for treatments to be comparable for research purposes, the same exercises are prescribed for both land and water-based programmes. However, it is possible that patients receiving aquatic-based exercises could tolerate more intense exercises, which could result in greater improvements than have currently been shown. Additionally, better compliance was found with the aquatic-based therapy groups (Foley et al, 2003; Lund et al, 2008; Silva et al, 2008), and less adverse effects were experienced (Lund et al, 2008). This may support the use of hydrotherapy as a more suitable treatment option than land-based exercise in patients with OA.
Published evidence on the benefits of hydrotherapy in OA within the canine field is extremely limited. Dini et al (2012) conducted a study on 12 dogs suffering from OA comparing two physical therapy protocols. The first group of dogs received treatment with laser therapy, magnetic therapy, treadmill exercise and electrical stimulation and the second group received treatment with diathermy and UWTM exercise. Treatments were applied three times per week for 4 weeks and response to treatment was measured via ROM, muscle mass circumference and evaluation of joint pain. Results showed that both treatment programmes led to improved ROM, increased muscle mass and reduced pain. The second group, which received diathermy and UWTM exercise, showed improvement in a shorter period of time than the first group, suggesting a more rapid benefit is experienced when using diathermy and UWTM. Unfortunately due to the combination of treatments that were administered it is not possible to determine the cause of the improvement and thus this study provides no conclusive evidence for the individual benefit of using UWTM treatment. A more recent study conducted by Nganvongpanit et al (2014) involving 55 dogs looked at the use of an 8 week swimming protocol on the function of the canine hip joint. The dogs were split into three groups: those with OA that followed the swimming protocol; those without OA that followed the swimming protocol; and those without OA that did not follow the swimming protocol. A significant (p<0.05) improvement was seen in lameness, joint mobility, weight bearing and pain on palpation in the OA group, along with significant improvements in the flexion and extension of the hip joint for both the OA group and the group without OA that completed the swimming sessions. Two serum biomarkers were also measured: hyaluronan (HA) and chondroitin sulphate epitope (CSE). In OA levels of HA have been shown to be reduced and CSE increased due to the pathogenic changes within the joint (Nganvongpanit et al, 2008). In the study conducted by Nganvongpanit et al (2014) following the 8 week swimming protocol, levels of HA had significantly increased in both osteoarthritic dogs and healthy dogs, and levels of CSE had significantly reduced in osteoarthritic dogs. The results of this study suggest that swimming had a positive impact on the functioning of the joint both in osteoarthritic dogs and those not suffering with OA.
Conclusion
Hydrotherapy has the potential to be highly beneficial to patients suffering with OA. It is already being used commonly within the UK which suggests that on an individual basis it is having a positive effect despite the lack of a substantial body of empirical evidence to support its use in canine patients. Although this article has concentrated on hydrotherapy, the reader should appreciate that it is advocated that the management of OA should take a multi-modal approach. Additional treatments may include analgesics, nutraceuticals, acupuncture and other physical therapeutics.
Key Points
- Osteoarthritis is a progressive condition which often results in pain and discomfort, and reduced activity levels.
- Maintaining mobility is important in osteoarthritic patients to maintain and improve joint range of motion and muscle strength, and reduce weight gain.
- Hydrotherapy is beneficial to osteoarthritic patients as it allows exercise to be conducted in a reduced weight bearing environment.
- Available evidence suggests that hydrotherapy is beneficial in the management of osteoarthritis.


