Nutritional support is a vital component of successful management of hospitalised dogs and cats. The provision of adequate caloric and nutrient intake is necessary to prevent adverse consequences of malnutrition and to optimise patient outcome. However, in a busy practice, Remillard et al (2001) have shown that it is easy for animals to receive suboptimal nutrition, because of vaguely written or absent feeding orders, anorexia, sedation from pain medications, procedures requiring the animal to be nil per os (NPO) or other factors that impede adequate calorie intake. Inadequate intake of calories and other nutrients can be deleterious to the animal's underlying illness or injury and can negatively affect outcome. The goals of this article are to review how to assess the nutritional needs of hospitalised dogs and cats, how to use that information to develop an individualised nutrition plan and how to best incorporate enteral nutrition into practice.
Why feeding is important to optimise outcome
When an animal does not receive adequate calories to meet energy requirements, amino acids are mobilised from lean body mass to compensate for that calorie deficit. This is the body's quick solution to the calorie deficit, but not a very efficient one since protein provides only 4 kilocalories (kcal) per gram compared with 9 kcal/g from fat. While healthy animals will make metabolic adaptations to allow fat to preferentially be used as an energy source and thus preserve lean body mass (i.e. simple starvation), ill or injured animals are not able to make this adaptive response and there is continued loss of lean muscle mass (i.e. stressed starvation or cachexia) (Chan and Freeman, 2006).
Additionally, while carbohydrates can be stored to a small extent as glycogen in the liver and there is a near unlimited capacity to store fat in adipose tissue, the body cannot effectively store protein. Since all of the body's protein is functional, loss of lean body mass means that the animal may be losing critical functional tissue. This loss of lean body mass in illness or injury has important clinical implications. In people, loss of lean body mass has been shown to impair strength, immune function, wound healing, and overall survival (Wolfe, 2006; Evans et al, 2008; Von Haehling et al, 2009). Therefore, it is critical to provide adequate calories, protein, and other nutrients to minimise the loss of lean body mass that occurs in ill or injured animals. In hospitalised dogs and cats, Brunetto et al (2010) have shown that lower body condition score (BCS) and energy intake were significantly associated with reduced survival, emphasising the importance of sufficient nutritional support.
Developing the plan
Optimal nutritional assessment will allow the clinician to develop an appropriate nutrition plan which will address patient selection, timing, route of administration and diet selection.
Patient selection
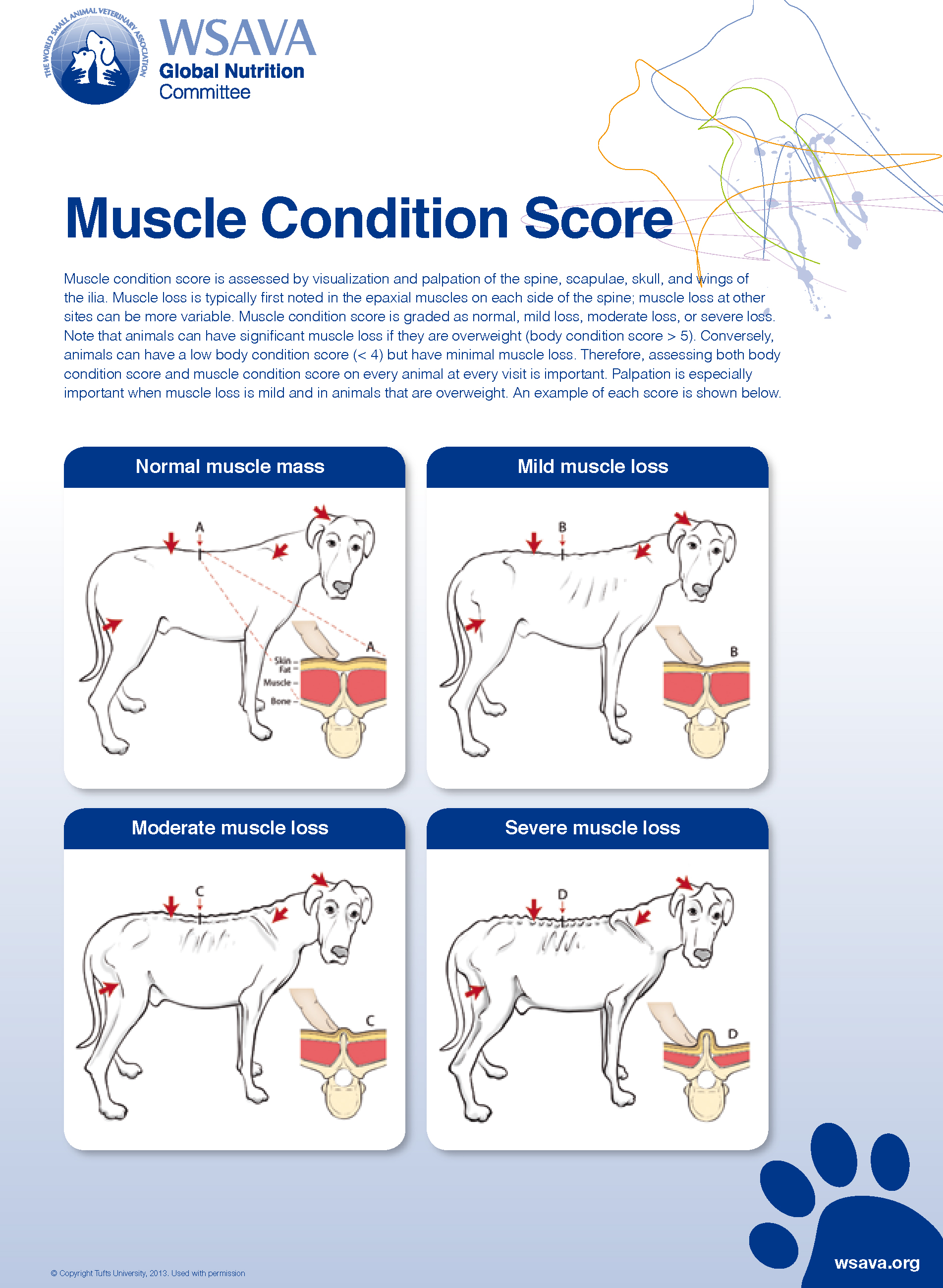
Guidelines developed by the American Animal Hospital Association (AAHA) (Baldwin et al, 2010) and the World Small Animal Veterinary Association (WSAVA) (Freeman et al, 2011) stress the importance of nutritional assessment in every patient at each visit. A nutritional screening includes reviewing the animal's life stage (e.g. growth, adult maintenance), bodyweight, BCS and muscle condition score (MCS), complete diet history, current medical conditions and medications. If any risk factors, such as weight loss, poor body condition (either thin or overweight), muscle loss or underlying disease are present, a more thorough diagnostic evaluation is recommended, such as laboratory testing and diagnostic imaging. Body condition primarily assesses fat stores while MCS assesses muscle. Body condition is determined by gently palpating over the ribs and assessing for the presence of a waist and abdominal tuck (Figures 1 and 2). Both 5-point and 9-point scoring systems are available for use. Regardless of which scoring system is used, it is important that the same system be used consistently among veterinary personnel. Muscle condition is determined by visual examination and palpation over the temporal bones, the scapulae, thoracic and lumbar vertebrae and the pelvic bones (Figure 3). It can be described as normal or can demonstrate varying degrees of atrophy (e.g. mild, moderate, marked) (Figure 4) BCS and MCS may not be analogous, as a thin animal may have normal muscle condition and an overweight, or even obese, animal may have significant muscle loss. Since animals with acute and chronic disease preferentially lose lean body mass (muscle), it is critical to assess both fat and muscle stores since this information can help to identify chronic disease and may modify the nutritional plan (Baldwin et al, 2010; Freeman et al, 2011).


When to feed
Factors that should be taken into account for selection of animals requiring assisted feeding include underlying medical conditions, medications and duration of anorexia (complete absence of appetite) or hyporexia (decreased appetite). Given the loss of critical lean body mass associated with illness or injury, animals that have been, or are anticipated to be, anorectic for longer than 3–5 days require nutritional support to prevent the risk of deleterious effects (e.g. enterocyte atrophy, decreased immune function) (Chan and Freeman, 2006; Baldwin et al, 2010; Freeman et al, 2011). In very young animals, this length of time may be too long to wait. As Remillard et al (2001) illustrated, it is important to consider a patient's entire duration of anorexia or hyporexia, including time at home prior to presentation. A feeding plan should be instituted as soon as the patient is haemodynamically stable. Whenever possible, enteral feeding is preferred because it supports normal gastrointestinal structure and function and is less expensive than parenteral nutrition.
Early nutritional support has been shown to improve outcome in veterinary patients. Puppies with parvovirus fed via nasoesophageal tubes showed earlier clinical improvement and significant weight gain compared with puppies that did not receive enteral nutrition prior to cessation of their vomiting (Mohr et al, 2003). Dogs with septic peritonitis that received nutritional support within 24 hours post operatively had a shorter length of hospitalisation compared with dogs that received delayed nutritional support (Liu et al, 2012).
Feeding plans
All nutrition plans should include precise feeding orders, including the specific diet, route, amount and frequency to be fed (e.g. Feed diet X orally — 10 g every 8 hours). The amount of food consumed and the caloric density of the food (i.e. calories per gram or mililiter; available from product guides, manufacturers’ websites, or by calling the manufacturer) must also be recorded to be able to monitor an animal's caloric intake. If the amounts fed and eaten are specifically documented in the record (e.g. Feed diet X orally — 10 g every 8 hours; ate 7 g), the specific number of calories eaten per day can be calculated and compared with the animal's requirements to ensure adequate voluntary intake. It is recommended to feed a hospitalised ill animal its resting energy requirement (RER). While the logarithmic formula for calculating RER, 70 x bodyweight in kg0.75, is more accurate, the linear equation, 30 x bodyweight in kg + 70, is a reasonable approximation of caloric needs for animals weighing 3–25 kg (Chan and Freeman, 2006). For animals that are at ideal weight (BCS 4–5/9), underweight (BCS <4/9), or mildly overweight (BCS 6/9), the animal's current weight should be used for calculating the RER. For animals that are moderately to severely overweight (BCS >7/9), an adjusted bodyweight may be more appropriate to calculate RER to avoid overfeeding. Also known as ‘permissive underfeeding’, this practice has been shown to reduce morbidity and mortality in ill obese people (Kushner and Drover, 2011). For animals with a BCS >7/9, the author uses the animal's ideal weight plus 25% of the animal's excess weight to account for the proportion of lean body mass present. Thus, for a 40 kg dog that should weigh 30 kg, the estimated weight for calculation of RER would be: 30 kg + (25% x 10 kg) = 32.5 kg
Once the animal is at home, maintenance energy requirement (MER) can be calculated by multiplying the RER by an activity factor. For an adult cat or dog, this MER activity factor may range from 0.8–1.8 x RER, depending on the animal's activity level and metabolism (Gross et al, 2010). However, it is important to remember that while these calculations for both RER and MER provide a starting point, the amount fed often needs to be adjusted over time to maintain bodyweight and optimal body condition. If weight loss is to be attempted, care should be taken to ensure no more than 1–2% bodyweight loss per week to avoid adverse consequences of rapid weight loss.
Appetite stimulants (e.g. mirtazapine) may provide some benefit to animals with decreased appetites (Plumb et al, 2011; Quimby et al, 2011a; Quimby et al, 2011b); however, the underlying disease must also be addressed. It is important to give specific feeding instructions and to monitor intake and bodyweight as many animals receiving appetite stimulants do not consume adequate amounts to achieve RER or to maintain weight.
If the animal does not eat sufficient amounts voluntarily to maintain weight, there are many methods by which animals can be encouraged to eat (Baldwin et al, 2010; Freeman et al, 2011). Coax feeding may be effective by offering the animal its favorite foods or feeding it in a quiet location. Warming the food or sitting with the animal may also increase food intake. Introducing a new diet to a hospitalised animal (especially a veterinary therapeutic diet indicated for long-term management) should not be prioritised during hospitalisation as it may contribute to the development of a food aversion (i.e. when the animal adversely associates a specific food with a feeling of illness). In some animals, syringe feeding may be attempted; however this is not routinely recommended.
Assisted enteral support
If oral intake is not possible, or if an animal will not voluntarily consume enough calories of a complete and balanced diet to meet its energy and nutrient requirements, assisted nutritional support is indicated. The decision to initiate assisted enteral support should be based on the animal's nutritional assessment (Baldwin et al, 2010; Freeman et al, 2011).
However, in general, assisted enteral support should begin at most within 3–5 days of the onset of reduced or absent appetite (including the period of time it was anorectic at home before hospitalisation). The optimal route for feeding in these animals depends on a number of patient factors, including the function of the gastrointestinal tract and the animal's ability to tolerate tube placement (e.g. anaesthesia, risk for pulmonary aspiration or other complications). Non-patient factors must also be considered, including cost and technical support.
Planning ahead for route of feeding before other procedures are performed is strongly recommended. If an animal with confirmed or anticipated anorexia is to be sedated or anaesthetised for a diagnostic or therapeutic procedure, placement of a feeding tube at that time should be considered. Prophylactic placement of a feeding tube can provide a ‘safety net’ when it is unclear whether or not the animal will begin eating on its own. In cases where the animal has intractable vomiting, severe malabsorption or neurologic disorders in which a gag response is compromised, parenteral nutrition is the preferred route of nutritional support. Parenteral nutrition is not without risk; additional information is available from other resources (Chan and Freeman, 2012; Perea, 2012).
Options for feeding tubes
There are a number of options for enteral feeding tubes. Factors to consider when choosing the most appropriate tube include the animal's nutritional status, ability to tolerate anaesthesia, the length of time the animal is expected to require nutritional support, hospital facilities, cost and the clinician's comfort level with different techniques for tube placement. Table 1 provides a comparison of various feeding tube options. Detailed information for placement of these feeding tubes is described elsewhere (Marks, 2010; Holahan et al, 2012; Larsen, 2012).
| Tube | Duration of nutritional support | Anaesthesia required (y/n) | Type of diet required | Other factors to consider |
|---|---|---|---|---|
| Nasoesophageal (NE) tube | Short term (<5 days) | No | Liquid | May cause epistaxis |
| Nasogastric (NG) tube | Short term (<5 days) | No | Liquid | May cause epistaxis; allows measurement of gastric residual volume and decompression of stomach |
| Esophagostomy (E) tube | Long term (weeks to months) | Yes | Critical care diet or canned food slurry | Well tolerated, not technically difficult to place |
| Gastrostomy (G) tube | Long term (weeks to months) | Yes | Critical care diet or canned food slurry | Requires surgical or endoscopic placement; early removal may have serious consequences |
| Jejuonostomy (J) tube | Short term (during hospitalisation) | Yes | Liquid | Early removal may have serious consequences |
Diet selection
Choosing the most appropriate diet depends on several factors. The animal's medical condition(s) should be a priority in diet selection. However, as previously stated, the diet chosen for long-term feeding may not be the most appropriate diet to begin feeding to a hospitalised animal given the risk of development of a food aversion. Non-patient factors, including diet availability and cost, are considerations. If an animal does not eat at least 50% of its calculated RER, supplemental nutrition may be provided via a feeding tube. If a feeding tube is used, this will influence appropriate diet selection as well.
For patients with small bore tubes (i.e., NE, NG and J tubes), a liquid enteral diet must be used. One liquid enteral diet that meets nutritional requirements for dogs and cats is CliniCare® Canine/Feline Liquid Diet (Abbott Laboratories). For cats with underlying renal disease that require controlled protein and phosphorus (or dogs requiring phosphorus restriction but minimal protein restriction), CliniCare® RF (Abbott Laboratories) is a better choice; however, this product provides too much protein for a dog that requires significant protein restriction. Not all enteral diets marketed for dogs and cats are nutritionally complete and balanced so they should be evaluated carefully to ensure that they meet the Association of American Feed Control Officials’ (AAFCO) minimums for all nutrients before use. Some human liquid enteral diets (e.g. Ensurec® (Abbott Nutrition), Peptamen® (Nestlé Healthcare Nutrition Inc)) can be used short term, but it is important to be aware that these diets do not provide complete and balanced nutrition, and modifications under the direct supervision of a board-certified veterinary nutritionist are necessary if they are to be used long term (i.e. >1 week). Some veterinary enteral ‘critical care’ diets are formulated to meet canine and feline nutritional requirements, have a consistency amenable to tube feeding, and are relatively calorie-dense. When mixed with a small amount of water (25 ml/156–170 g can), these diets provide 1.0–1.8 kilocalories (kcal) per ml (Table 2). Each diet has a different nutrient profile which can be used to determine the diet most appropriate for an individual animal (e.g. avoiding diets high in sodium for a cat with cardiac disease or selecting a diet higher in protein for a young, growing puppy).
| Diet | Kcal per can | Water added (ml per can) | Kcal per ml (slurry) | Protein (g/100 kcal) | Fat (g/100 kcal) | Sodium (mg/100 kcal) |
|---|---|---|---|---|---|---|
| Iams Veterinary FormulaTM Maximum-Calorie PlusTM(P&G Pet Care) (170 g can) | 333 | 25 | 1.8 | 7.2 | 6.4 | 33 |
| Hill's Prescription Diet® a/d® Canine/Feline Critical Care (156 g can) | 180 | 25 | 1.0 | 9.2 | 6.3 | 165 |
| Royal Canin Veterinary Diet® Recovery RSTM (164 g can) | 180 | 25 | 1.0 | 9.9 | 6.5 | 210 |
When these critical care diets are contraindicated for an individual animal (e.g. a dog with advanced kidney disease or a cat requiring a novel ingredient diet), commercial canned veterinary diets may be blended with water to provide appropriate nutritional support for enteral feeding. The amount of water that must be added to each diet varies based on the individual diet, but the resulting caloric density is typically well below that of the recovery diets (e.g. 0.5–1.2 kcal/ml). Additionally, these diets require 2–3 minutes of high speed blending before administration and require careful flushing to avoid tube clogging.
Conclusion
There are many ways to provide nutritional support to ill or injured animals. The key is to perform a thorough nutritional assessment of every animal and to initiate nutritional intervention early. Early institution of an individualised nutritional plan allows enhanced nutritional management, reduced risk of malnutrition and ultimately improved patient outcome.

