Opioids have been used as analgesics in human and veterinary medicine for many years. Papaverum somniferum (the opium poppy) is the origin of all opioids. This particular species of poppy has been used to provide analgesia by a variety of means (Kerr, 2007; Lamont et al, 2007; Dugdale, 2010). Opioid analgesics are the most effective and versatile group of drugs with extensive application in the pain management of acute trauma patients, patients undergoing surgical and/or diagnostic procedures, patients with painful medical conditions or disease processes, and in patients suffering from chronic pain, e.g. cancer pain, requiring longterm analgesic therapy (Lamont and Mathews, 2007; Gurney, 2012).
Opioids are commonly used in veterinary patients and, as such, it is essential that veterinary nurses understand how this class of drugs may affect patients. This article will provide detailed information on how opioids provide analgesia and focus on potential side effects, drug interactions and in which circumstances a particular opioid should be chosen.
The International Association for the Study of Pain defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage’ (Covin 2007; Dugdale 2010). In veterinary patients, however, pain can be defined as a complex unpleasant sensory and emotional experience where actual or potential tissue damage may be present producing a change in physiology and behaviour directed to reduce or avoid the damage, reduce the likelihood of recurrence and promote recovery (Kerr, 2007). Pain is made all the more difficult to treat in animals due to their inability to verbalise the extent of their discomfort as well as the adequacy of treatment in comparison to human patients. Inadequate treatment of pain can lead to a decrease in quality of life, prolonged recovery time from surgery, injury and illnesses. Secondary effects such as loss of appetite and self trauma may occur, which also contributes to prolonged recovery from the underlying condition (Dohoo and Dohoo, 1996; Hewson et al, 2006; Hellyer et al, 2007).
Preventing and managing pain has become a fundamental part of care in veterinary medicine, and important concepts have been adopted in the management of pain such as pre-emptive, preventive and multimodal analgesia (Hellyer et al, 2007; Flaherty, 2013):
- Pre-emptive analgesia — defined as the administration of analgesic drugs prior to the onset of noxious stimulation which has the potential to be more efficacious. Immediate post-operative pain may be reduced, any subsequent pain experienced is of lesser intensity and duration, and may be easier to control with analgesics because the initiation and establishment of peripheral and central sensitisation is potentially reduced (Dahl and Moiniche, 2004; Dugdale, 2010; Flaherty, 2013).
- Multimodal analgesia — combining different analgesics acting by different mechanisms or at different sites in the nervous system. This leads to additive and improved analgesia, while simultaneously minimising the overall side effects for the patient by reducing the dose of each individual drug (Buvanendran and Kroin, 2009; Dugdale, 2010; Flaherty, 2013).
- Preventive analgesia — combines multimodal and pre-emptive concepts but continues into the early post-operative period (Dugdale, 2010; Flaherty 2013).
Regulation of opioids
In the UK, opioids are classified as ‘controlled drugs’ due to their propensity for abuse. This means that the purchase, storage and use of opioids are legally regulated. The degree of regulation is dependent on the Schedule (Kerr, 2007; Dugdale, 2010). The majority of opioids used in veterinary medicine are classified as Schedule 2 drugs. The implications of this are as follows:
- Requisition requires a prescription.
- They must be stored in a lockable cupboard which meets specific criteria.
- When prescribed and dispensed, this must be recorded in a controlled drug register which must be bound. The register must be kept for 2 years after the date of the last entry.
- Destruction of stock controlled drugs (e.g. out of date) must be witnessed by a person authorised by the Secretary of State (Police Officer or Practice Standards Scheme Inspector) or a member of the Royal College of Veterinary Surgeons (MRCVS) with no association with the practice. Ideally this should not be a locum.
Schedule 3 opioids are still subject to requisition requirements and the only relevant opioid in veterinary practice — buprenorphine — must be kept in a locked cabinet. However, dispensed buprenorphine does not need to be recorded in a register. Butorphanol is not a controlled drug in the UK although it may be classed as such in other countries.
Opioids are available in single or multi-dose vials. Single dose vials have the advantage of being prescribed and dispensed to a single patient which makes record keeping simpler. Multi-dose preparations from which doses can be dispensed for several patients makes record keeping more difficult due to needle hub and syringe dead space. This can lead to discrepancies in the running balance when a bottle is finished.
Definitions and classification
The term opiate refers to drugs derived directly from opium and includes morphine, codeine and a variety of related alkaloids (Covin, 2007; Lamont and Mathews, 2007; Gupta et al, 2011). The term opioid is used broadly and includes any naturally occurring, semisynthetic or synthetic substance with morphine-like activity that act at opioid receptors (Covin, 2007; Hellyer et al, 2007; Kerr, 2007; Gupta et al, 2011).
Opioids are often referred to as narcotic analgesics as they provide analgesia but can also induce a state similar to sedation or euphoria. In pharmacology, narcosis refers to a state of sleep, but this term may be applied to any substance that has the potential to cause dependence (Hsu and Riesdesel, 2008; Dugdale, 2010; Gupta, 2011).
Drugs that act at opioid receptors may be classified as full agonists, partial agonists, agonist-antagonists or antagonists at opioid receptors. The term ‘agonist’ can be defined as a chemical that binds to receptor and triggers an effect in the associated cell, whereas an antagonist blocks that effect. Partial agonists are substances that activate the receptor but only produce a partial physiological response compared with a full agonist. The partial response is due to lower intrinsic activity (Dugdale 2010, Hsu et al 2008 and Vaughn et al 2012).
Affinity may be defined as how well or how tightly a drug binds to its receptor but does not indicate what the effect will be. For example, morphine (agonist) and naloxone (antagonist) have the same affinity for the μ-receptor, however they have different effects.
Potency is a measure of relative drug activity that is inversely related to the concentration required to produce a standard effect. Drug potency can be described as the relationship between the amount of drug administered and its effect, i.e. a more potent opioid will have a lower dose requirement to produce the desired effect (Hellyer et al, 2007; Lamont and Mathews, 2007). A ‘ceiling effect’ refers to a plateau effect in the level of analgesia despite increased doses (Figure 1) (Kerr, 2007; Dugdale, 2010).
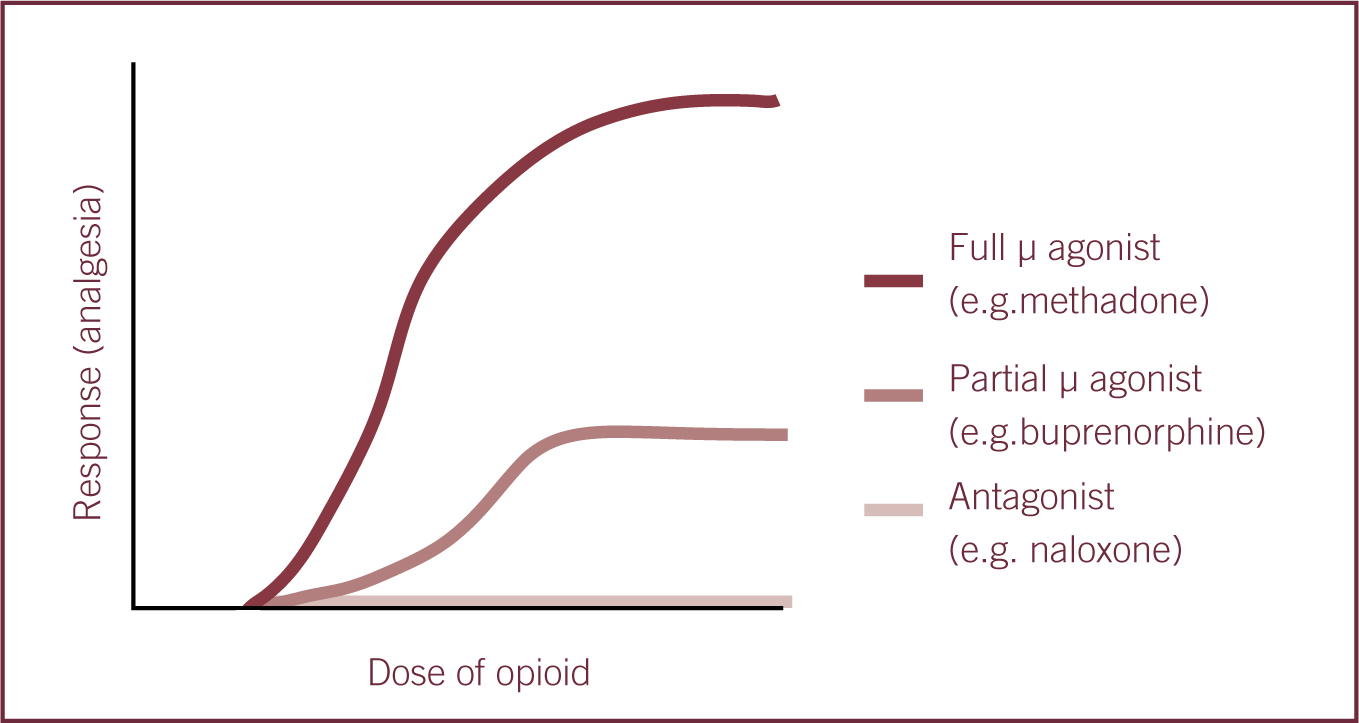
Tolerance can occur in patients receiving long-term pain treatment, for example, treatment for malignant and chronic non-malignant pain, and it can be defined as an increase in the dose of a given drug in order to attain a similar effect. Long-term use of opioids has been reported to lead to dependence or addiction in human patients. This is the compulsive need to use drugs in order to function normally (Wikler, 1973). When there is an abrupt cessation of opioids, this can lead to withdrawal symptoms. Withdrawal may also occur in veterinary patients following long-term opioid treatment but is difficult to recognise (Wikler, 1973; Hellyer et al, 2007; Dugdale, 2010).
Patients receiving opioid treatment may become more sensitive to pain as a direct result of opioid therapy. This phenomenon is known as opioid-induced hyperalgesia (Angst et al, 2006; Mercadante, 2006; Dugdale, 2010).
Sequential analgesia is a technique that involves the administration of an agonist-antagonist or a partial agonist (e.g. butorphanol or buprenorphine) which antagonises the respiratory or sedative effects of a full agonist (e.g. methadone), while facilitating retention of appropriate analgesia (Hsu and Riedesel, 2008; Dugdale, 2010; Vaughn et al, 2012). In the authors' experience, remarkably low doses of butorphanol can be titrated intravenously to effect in patients which are experiencing unacceptably long recoveries following the administration of full agonists, e.g. patients that have undergone lengthy procedures during which fentanyl has been infused. This technique may also be useful in animals receiving relative overdoses of methadone, particularly if it is desirable that analgesia is maintained.
Pharmacology
All opioid receptors are found throughout the central nervous system (CNS) in somatic and visceral sensory neurons, and in the periphery (e.g. gastrointestinal tract and joints), especially after inflammation, and all mediate inhibition of pain. There are three key opioid receptor types, most commonly known by their Greek letter descriptions as μ (mu), κ (kappa) and δ (delta). These receptors may also be characterised as MOP, MOR or OP3 (mu), KOP, KOR or OP2 (kappa) and DOP, DOR or OP1 (delta) (Kerr, 2007; Lamont and Mathews, 2007; Taylor and Clarke, 2007; Dugdale 2010). Opioid receptors belong to the G-protein coupled family of receptors. Activation results in changes in enzyme activity such as adenylate cyclase or alterations in calcium and potassium ion channel permeability resulting in hyperpolarisation of the cell membrane. Receptors are found throughout the brain and in the dorsal horn of the spinal cord. Activation of μ-receptors produces supraspinal and spinal analgesia, euphoria, sedation, miosis (e.g. dogs), mydriasis (e.g. cats), respiratory depression, and decreased gastrointestinal motility due to inhibition of acetylcholine (ACh). Activation of κ-receptors results in spinal and supraspinal analgesia, mild sedation, dysphoria, diuresis and miosis. Activation of δ-receptors results in spinal and supraspinal analgesia and cardiovascular depression (Lamont and Mathews, 2007; Hsu and Riedesel, 2008).
Generally, μ-agonists produce more efficacious analgesia and are recommended for moderate to severe pain as well as procedures where noxious stimulation will be high; a κ-agonist will produce better sedation and have been recommended for superficial and visceral pain relief (Benson, 2002; Kerr, 2007; Lamont and Mathews, 2007; Shaffran, 2008; Hurley and Adams, 2011; Vaughn et al, 2012). In the authors' experience, a κ-agonist has a better sedative effect than analgesic when given alone compared with μ-agonists and are usually used as part of premedication for non-painful procedures, e.g. diagnostic imaging. For minor surgical procedures where nociception is anticipated to be mild, partial μ-agonists would be appropriate and would provide suitable analgesia.
Opioid receptor distribution varies between species suggesting that the response to opioids will differ. Dose requirements may also be significantly different. For example, μ-agonist opioids in humans tend to cause narcosis whereas in horses and cats, they cause increased locomotor stimulation and excitement, especially if given in the absence of pain. This difference in response appears to be due to the fact that horses and cats have fewer μ receptors in the CNS compared with humans and so correspondingly require much lower doses. Birds and reptiles have an increased distribution of κ receptors compared with other species like dogs and cats, and analgesia appears superior when administered κ-agonist opioids, although pain recognition in these species is challenging (Benson, 2002; Taylor and Clarke, 2007; Hsu and Riedesel, 2008; Dugdale, 2010).
Human patients demonstrate clinical variation in response to treatment with opioids, which has been shown to have a genetic basis. Each patient has a unique genetic background, which may influence how that patient responds pharmacokinetically and pharmacodynamically to any drug. As such analgesic regimens need to be individualised for each patient to maximise pain relief and minimise side effects. Opioid switching or rotation has been suggested as a good approach in patients whose current opioid is ineffective or in those patients experiencing unacceptable side effects (Argoff, 2010; Mayer et al, 2006).
Effects of opioids
Clinically, opioids are primarily indicated for their analgesic properties and are considered more effective for continuous dull pain rather than sharp intermittent pain. They are the most efficacious drugs for severe and chronic pain, but respiratory depression, nausea, constipation, addiction and tolerance are some of the side effects associated with their use (Neal, 2002; Stein et al, 2003; Covin, 2007; Kerr, 2007; Lamont and Mathews, 2007; Hsu and Riedesel, 2008; Dugdale, 2010).
Central nervous system (CNS) effects
Arousal
- CNS depression is characterised by sedation and is typically seen in dogs, monkeys and humans. CNS stimulation characterised by excitement and/or spontaneous locomotor stimulation may occur in cats, horses and ruminants (Lascelles and Waterman, 1997; Molony and Kent, 1997; Valverde and Gunkel, 2005).
- Opioids can occasionally produce dysphoria and this can be characterised by agitation, excitement, restlessness, excessive vocalisation and disorientation in dogs. In cats, dysphoria is described by fearful behaviour, open-mouth breathing, agitation, vocalisation, pacing and apparent hallucinations. These signs are often mistaken for pain.
- μ-agonist opioids tend to produce euphoria. In dogs, it is described as excessive wakefulness and vocalisation. In cats, it produces rolling, grooming, ‘kneading’ and extreme friendliness.
- Both dysphoria and euphoria can be observed when high doses of opioids are administered and they can be managed by administering sedatives/tranquilisers and/or opioid antagonists or by the use of sequential analgesia as described above.
Thermoregulation centre
- The hypothalamic thermoregulatory centre is affected by opioid administration. Hypothermia tends to be the most common response.
- In dogs, opioids decrease the thermoregulatory set point in the CNS and cause panting but this effect tends to decrease with the onset of hypothermia.
- Hyperthermia may be seen in some clinical circumstances in cats, horses, ruminants and swine. Part of this increase in body temperature may be due to an increase in muscle activity associated with CNS excitation in these species (Molony and Kent, 1997; Valverde and Gunkel, 2005).
Respiratory effects
- Opioids produce depression of ventilation, caused by a depressant effect on the brain-stem respiratory centre, i.e. a reduced response to hypercapnia and hypoxaemia, and this effect is dose dependent.
- The combination of opioids with other CNS depressants such as sedatives, tranquilizers, hypnotics and volatile anaesthetic agents will contribute to significant respiratory depression and hypercapnia, therefore patients should be closely monitored for apnoea or severe hypoventilation.
- Cough centre:
- ∘ Cough suppression depends on the individual opioid rather than receptor affinity. This is partly by a direct effect on the cough centre located in the medulla.
- ∘ Butorphanol and fentanyl are considered effective antitussives.
Cardiovascular effects
- Most opioids have minimal effects on the cardiovascular system at clinical doses. This class of drugs is the preferred choice for geriatric and critical patients due to its minimal cardiovascular effects (Dyson, 2008a and 2008b; Baetge and Matthews, 2012; Quandt, 2013).
- Bradycardia may be observed following moderate to high doses of opioids. This is secondary to increased vagal tone and is usually responsive to anticholinergic treatment.
- Some opioids, e.g. morphine and pethidine, are known to cause histamine release, especially after rapid intravenous administration, which may lead to vasodilation and hypotension. The administration of pethidine via the intravenous route is contraindicated.
- Pethidine has activity at non-opioid receptors and this can affect the cardiovascular system differently. Pethidine has a similar structure to atropine so pethidine tends to increase heart rate rather than predispose patients to bradycardia.
- Care should be taken when administering opioids intravenously, in particular μ-receptor opioids. If an intravenous route is chosen, it is advised to dilute the dose and administer slowly as fast injections may increase chances of cardiovascular side effects.
Gastrointestinal effects
- Emetic centre:
- ∘ Nausea and vomiting associated with opioid administration are caused by direct stimulation of the chemoreceptor trigger zone (CTZ) for emesis located in the medulla oblongata.
- ∘ As with the other centrally mediated side effects, species plays a role in determining an individual's tendency to vomit after an opioid is administered. In swine, vomiting does not occur with opioid administration. Cats may vomit but usually at doses that are greater than those that stimulate vomiting in dogs. Dogs may vomit following the administration of morphine.
- In patients experiencing pain or when opioids are administered in the immediate post-operative period, vomiting is uncommon. Decrease in gastric emptying time and intestinal propulsive motility is mediated by μ and δ receptors. This may be more relevant in horses, which may be prone to developing gastrointestinal impaction and colic.
- The sphincter of Oddi — a combined sphincter controlling the flow of bile and pancreatic juices into the duodenum — may also constrict following the administration of morphine, and some clinicians avoid its use in cases where this may be detrimental, e.g. pancreatitis. However, although this may be true in the cat, the vast majority of dogs have a separate pancreatic sphincter and the administration of morphine may not be detrimental in this species (Hershey et al, 1984; Flaherty, 2009; Thompson, 2001).
Ocular effects
- As a general rule, μ-agonist opioids produce miosis in species that exhibit CNS depression such as dogs, but produce mydriasis in those that exhibit CNS excitation, e.g. cats and horses. Perhaps due to mydriasis, these species are more likely to be sensitive to light therefore exhibiting CNS excitation (Lascelles et al, 1997; Quandt, 2013).
- Some ophthalmologists may require the pupils to be dilated for selective intraocular procedures, such as phacoemulsification. In such cases, the miotic effect of opioids produced by excitatory action of opioids in the oculomotor nuclear complex is not ideal. Pethidine, because of its similar structure to atropine, does not have the same miotic effect as the other opioids and mydriasis seems to occur instead (Kerr, 2007). This may make it the opioid of choice for intraocular procedures, but it must be administered at appropriate intervals as its duration of effect is short.
Urinary effects
- Opioids can cause urinary retention by increasing urethral sphincter tone and decreasing bladder detrusor tone, leading to a decreased awareness of bladder distension and inhibition of the reflex urge to void. In particular this may be observed when opioids are administered neuroaxially (i.e. via epidural or spinal routes) (Kerr, 2007; Lamont et al, 2007).
- Manual bladder expression or urinary catheter placement may be required in certain patients until normal function returns.
Conclusion
Opioids continue to be the cornerstone of effective pain treatment in veterinary medicine, especially in small animal practice. They are versatile group of drugs with extensive applications in the management of pain in patients with acute trauma, in patients undergoing surgical procedures, in patients with medical conditions or disease processes, and in patients suffering from chronic pain that require long-term therapy. This type of patient often requires intensive nursing and therefore it is imperative that the veterinary nurse is conversant with the management of patients receiving opioids and is able to recognise inappropriate analgesia and potential side effects, which may result from their administration. It is likely that more veterinary practices will have Schedule 2 opioids available for administration to patients suffering from moderate to severe pain as more drugs become licensed in veterinary species. Therefore, it is essential that the practice team are aware of the legislative issues surrounding the purchase, storage and use of opioids but this should not dissuade from their use.
Key points
- Opioids, often in combination with other classes of drugs, form the basis of pain management in veterinary practice.
- Opioids are classified as controlled drugs and their use is strictly regulated in the UK.
- There are three main opioid receptor types commonly known as μ (mu), (kappa) and δ (delta).
- Drugs that act at opioid receptors can be classified as full agonists, partial agonists, agonist-antagonists or antagonists.
- The veterinary nurse must be conversant with the side effects of opioids in order to be able to provide appropriate care and management for patients.

